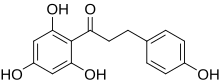
Phlorizin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phlorizin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 24 O 10 | ||||||||||||||||||
| Brief description |
white, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 436.4 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
106-109 ° C |
||||||||||||||||||
| boiling point |
decomposition |
||||||||||||||||||
| solubility |
poor in water (1 g l −1 at 22 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phlorizin (also phloridzin , phlorrhizin ) is a vegetable glycoside from the group of flavonoids ( chalcones ), which is formally derived from phloroglucin .

Phlorizin is found in the bark of pears ( Pyrus communis ), apples , cherries and other fruit trees ( Rosaceae ). The aglycon of the substance is phloretin .
properties
The crystalline, white to yellowish, sweet-tasting substance contains four molecules of crystal water and melts at 106-109 ° C. From around 200 ° C it decomposes to rufin. It dissolves poorly in cold water and ether , better in hot water and ethanol . With prolonged exposure to aqueous acid solutions , phlorizin hydrolyzes to phloretin and glucose.
Effect and use
Phlorizin and phloretin block specifically the glucose - absorption by the renal tubules by inhibition of the sodium / glucose cotransporter SGLT1 what a glucosuria ( Phlorizindiabetes causes). Phlorizin was previously used as a substitute for quinine and in experimental physiology.
Individual evidence
- ↑ entry to phloridzin in CosIng database of the European Commission, accessed on 7 April 2020th
- ↑ Phlorizin data sheet (PDF) from Carl Roth , accessed on March 9, 2010.
- ↑ a b Ch. Gerhardt (Ed.): Textbook of Organic Chemistry , 1857, Wigand-Verlag.
- ↑ a b Entry on phlorizin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b data sheet phloridzin dihydrate from Sigma-Aldrich , accessed on April 19, 2011 ( PDF ).
- ↑ Summary Tables of Biological Tests. National Research Council Chemical-Biological Coordination Center. Vol. 6, p. 226, 1954.