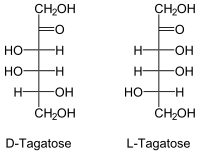
Tagatose
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| Fischer projection , open-chain representation | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname |
|
|||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 12 O 6 | |||||||||||||||||||||
| Brief description |
White dust |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 180.16 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
129-133 ° C |
|||||||||||||||||||||
| solubility |
soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tagatose is a monosaccharide with six carbon atoms. This sugar belongs to the group of ketohexoses . D- Tagatose is suitable as a sweetener because - compared to fructose - with 92% sweetness it has only 38% of the physiological calorific value .
As with any sugar (except for dihydroxyacetone ) there are two enantiomeric forms that behave like an image and a mirror image.
Occurrence
D- tagatose occurs naturally in some dairy products, albeit in small amounts.
Extraction and presentation
Commercially, D- tagatose is obtained from lactose . The disaccharide is first hydrolytically split into glucose and galactose . After the mixture has been separated, the galactose is isomerized to D -tagatose under alkaline conditions .
properties
An intramolecular ring closure occurs in aqueous solution, so that an equilibrium is established between the keto form (less than 1%) and the two ring forms ( furanose form and pyranose form). The position of the equilibrium depends on the temperature and also influences the sweetening power of the sugar. At 27 ° C the following equilibrium is established:
- α-pyranose form: 79% β-pyranose form: 16%
- α-furanose form: 1% β-furanose form: 4%
D -Tagatose - spellings Wedge formula Haworth notation 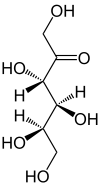

α- D -tagatofuranose
β- D -tagatofuranose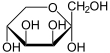
α- D -tagatopyranose
β- D -tagatopyranose
In studies with test persons it could be shown that D- tagatose is not cariogenic and therefore tooth-friendly. Compared to glucose, it shows a very low insulin response . In larger quantities it has a laxative effect.
use
D -Tagatose is in the diet -Trinkeis Diet Slurpee company PepsiCo exclusively for the American market in 7-Eleven offered shops. In the USA there is also approval for use as a coating agent ("freezing") for breakfast cereals containing bran , where it is of technological interest due to the high crystallization speed . It can be used as the sole sweetener in chocolate . In Europe it was approved as a novel food in 2005 .
Individual evidence
- ↑ a b c d e data sheet D - (-) - Tagatose at AlfaAesar, accessed on December 23, 2019 ( PDF )(JavaScript required) .
- ↑ a b Kurt Rosenplenter, Gert-Wolfhard of Rymon Lipinski, Ulrich Nöhle : Manual sweeteners. Behr's Verlag , 2007, ISBN 978-3-89947-947-8 .
- ↑ Joachim Bröckel: EU cartel watchdogs approve SweetGredients. In: Accents. January 2004, Nordzucker
- ↑ Public consultation: Conversion factor for energy value of D-Tagatose for labeling purposes , European Food Safety Authority , July 18, 2016.
Web links
- WLP Bredie, S. Bachmann, SMB Johansen, G Hansen: The sweetness of D -tagatose - a novel food ingredient. ( Memento from November 15, 2007 in the Internet Archive )