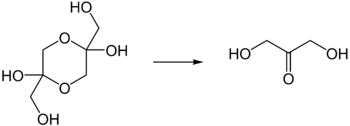Dihydroxyacetone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dihydroxyacetone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 6 O 3 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 90.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
75–80 ° C (mixture of monomer and dimer) |
||||||||||||||||||
| solubility |
good in water (930 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dihydroxyacetone (abbreviated DHA , glycerone ) is a simple carbohydrate with the empirical formula C 3 H 6 O 3 . Since dihydroxyacetone does not have a stereocenter, it is actually not considered a monosaccharide . However, it is essential to the metabolism of carbohydrates. DHA is the essential ingredient in self-tanners and reacts with proteins in the horny layer - the top layer of skin, which turns brown in the process.
presentation
Dihydroxyacetone, similarly to the glyceraldehyde through oxidation of glycerol with mild oxidizing agents such as dilute hydrogen peroxide solution, in the presence of iron salts as catalyst , are prepared. Large-scale production takes place biotechnologically through the microbial fermentation of glycerine by Gluconobacter oxydans in an order of magnitude of around 2,000 t per year worldwide. An alternative to this relatively complex technology is the anodic oxidation published in 2007 in the presence of the catalyst 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO).
properties
Dihydroxyacetone is a white, hygroscopic powder with a characteristic fragrance and sweet taste. It belongs to the group of sugars and within this group, because of the three carbon atoms that make up the molecule, to the trioses . As the simplest conceivable ketosis , the molecule has no center of chirality and is optically inactive . Dihydroxyacetone is usually present in the solid state as a dimer which, when dissolved in water, rapidly cleaves into the monomer . This reaction proceeds according to a time law of the first order. The half-life at room temperature is 20.4 minutes.
Biological importance
Of particular importance in biochemistry is a phosphoric acid ester of dihydroxyacetone, dihydroxyacetone phosphate (shown here as a dianion , as it usually occurs under physiological conditions).
Its importance in metabolic physiology is that it is isomerized to the important glyceraldehyde-3-phosphate . This isomerization takes place with base catalysis via the Lobry-de-Bruyn-Alberda-van-Ekenstein rearrangement . In the following figure, the residue - R stands for the group –CH 2 –OH:
Like their phosphoric acid esters , dihydroxyacetone and glyceraldehyde are isomeric to one another . They are in chemical equilibrium due to the rearrangement shown above . In the cell , these equilibrium reactions are catalyzed by certain enzymes . In bacteria, dihydroxyacetone is produced by glycerol dehydrogenase .
Web links
Individual evidence
- ↑ a b c d e data sheet dihydroxyacetone (PDF) from Merck , accessed on December 20, 2018.
- ↑ Mario Pagliaro, Rosaria Ciriminna, Hiroshi Kimura, Michele Rossi, Christina Della Pina: From Glycerin to Higher Quality Products , in: Angewandte Chemie , 2007 , 119 , pp. 4516–4522 ( doi : 10.1002 / anie.200604694 ).
- ↑ Leodis Davis: The structure of dihydroxyacetone in solution , in: Bioorg. Chem. , 1973 , 2 , pp. 197-201 ( doi : 10.1016 / 0045-2068 (73) 90023-0 ).