Idoses
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
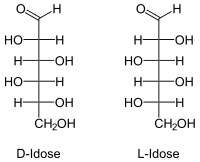
| Surname | D - (-) - idose, L - (+) - idose | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 O 6 | ||||||||||||||||||
| Brief description |
colorless syrup |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 180.16 g mol −1 | ||||||||||||||||||
| Physical state |
solid, technically often liquid |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Idose is a monosaccharide with six carbon atoms. This sugar belongs to the group of non-naturally occurring aldohexoses .
By oxidation of the terminal CH 2 OH group on the carboxy group (-COOH) which arises iduronic acid (a uronic acid ). This is a component of the two glycosaminoglycans dermatan sulfate and heparan sulfate .
As with any sugar (except for dihydroxyacetone ) there are two isomeric forms that are mirror images of each other ( enantiomers ). Whenever “Idose” is mentioned in this text or in the scientific literature without any additional name ( prefix ), D -Idose is meant. L -Idose is of only marginal importance.
properties
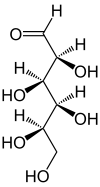
In aqueous solution, an intramolecular ring closure sometimes occurs, so that an equilibrium is established between the aldehyde form and the two ring forms ( furanose and pyranose ):
| D -Idose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |
|
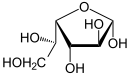 α- D -idofuranose 16% |
 β- D -idofuranose 16% |
 α- D -idopyranose 31% |
 β- D -idopyranose 37% |
|
Web links
Individual evidence
- ↑ a b Entry on Idose. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Jürg Hunziker: Carbohydrate Chemistry ( Memento from May 10, 2008 in the Internet Archive ), April 1, 2007.