Sorbose
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
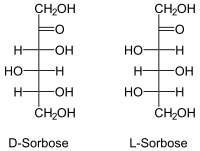
| Surname | D - (+) - sorbose, L - (-) - sorbose | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 O 6 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 180.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
163-165 ° C |
||||||||||||||||||
| solubility |
550 g l −1 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sorbose is a monosaccharide with six carbon atoms. This sugar belongs to the group of ketohexoses .
L- Sorbose is an intermediate product in the synthesis of vitamin C according to Reichstein . In this process, glucose is first reduced to sorbitol and then oxidized to L -sorbose with sorbose bacteria . The L- sorbose is further oxidized with the addition of acetone , with vitamin C being formed after the acetone is subsequently split off and water is split off.
properties
As with any sugar (except for dihydroxyacetone ) there are two enantiomeric forms that behave like an image and a mirror image.
| D -Sorbose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
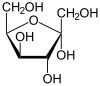 α- D -sorbofuranose |
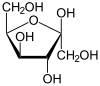 β- D -sorbofuranose |
 α- D -sorbopyranose |
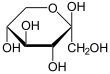 β- D -sorbopyranose |
|
In aqueous solution there is sometimes an intramolecular ring closure, so that an equilibrium is established between the keto form and the two ring forms ( furanose form and pyranose form), with sorbose then being almost exclusively in the pyranose form.
literature
- Z. Li, Y. Gao, H. Nakanishi, X. Gao, L. Cai: Biosynthesis of rare hexoses using microorganisms and related enzymes. In: Beilstein journal of organic chemistry. Volume 9, 2013, pp. 2434-2445, doi : 10.3762 / bjoc.9.281 . PMID 24367410 . PMC 3869271 (free full text).
- J. Hirabayashi: On the origin of elementary hexoses. In: The Quarterly review of biology. Volume 71, Number 3, September 1996, pp. 365-380, PMID 8927690 .
Web links
Individual evidence
- ↑ a b c d e Entry on L- Sorbose in the GESTIS substance database of the IFA , accessed on February 5, 2018(JavaScript required) .