Reichstein synthesis
The Reichstein synthesis is a combined chemical and microbiological process for the production of ascorbic acid from D- glucose , which takes place in several steps. It was developed by Nobel Prize winner Tadeus Reichstein and his colleagues Andreas Grüssner and Rupert Oppenauer in 1933 in the laboratories of the ETH in Zurich .
Reaction steps
The individual reaction steps are:
- Hydrogenation of D - glucose to D - sorbitol as an initial reaction (a purely chemical reaction) with nickel as a catalyst under high temperature and high pressure
- Microbiological oxidation ( fermentation ) of D -Sorbitol to L - sorbose (with Acetobacter ) at pH 4-6 and 30-35 ° C
- Protection of 4 hydroxyl groups of L-sorbose by reaction with acetone and acid to form diacetone-L-sorbose (2,3: 4,6 − diisopropylidene − α − L − sorbose)
- Oxidation with alkaline potassium permanganate solution produces a carboxylic acid , diacetone-2-keto- L- gulonic acid
- Removal of the protective groups by heating with water gives 2-keto- L -gulonic acid
- In a last reaction step , ring closure takes place (γ-lactonization with elimination of water) to L -ascorbic acid.
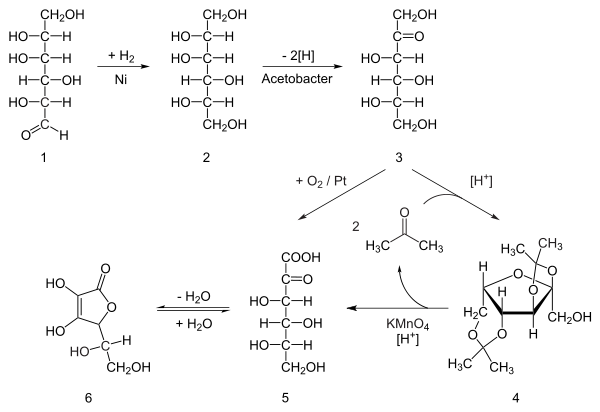
The microbiological oxidation of D -sorbitol to L -sorbose is necessary for stereochemical reasons.
meaning
The synthesis was patented and sold to Hoffmann-La Roche in 1933 . The first commercially produced vitamin C product was the Cebion of Merck .
Even today, all large-scale processes for the production of ascorbic acid are more or less based on the synthetic route discovered by Reichstein.
However, the direct oxidation of sorbose to 2-keto-L-gulonic acid, e.g. B. at platinum -carrier catalysts (according to Kurt Heyns , 1942). The laborious introduction of protective groups and their subsequent removal is avoided. This synthesis route produces 5-keto-D-gluconic acid as a by-product. There are also various ways of microbial synthesis, and genetically modified strains have recently allowed one-step fermentation .
Individual evidence
- ↑ T. Reichstein, A. Grüssner, R. Oppenauer: The synthesis of d-ascorbic acid (d-form of the C vitamin). In: Helv. Chim. Acta . 16, 1933, pp. 561-565, doi: 10.1002 / hlca.19330160177 .
- ↑ H. Trommer, R. Böttcher, RHH Neubert: Ascorbic acid - a vitamin like Dr. Jekyll & Mr. Hyde . In: Pharmaceutical Newspaper Online. 2002.
- ^ Wittko Francke , Wolfgang Walter : Textbook of organic chemistry. 24., revised. Edition. S. Hirzel Verlag, Stuttgart 2004, ISBN 3-7776-1221-9 , p. 480.
- ↑ T. Reichstein, A. Grüssner: A rich synthesis of L-ascorbic acid (C vitamin). In: Helv. Chim. Acta . 17, 1934, pp. 311-328, doi: 10.1002 / hlca.19340170136 .
- ^ C. Brönnimann et al .: Direct oxidation of L-sorbose to 2-Keto-L-gulonic acid with molecular oxygen on platinum and palladium-based catalysts. In: J. Catal. 150 (1), 1994, pp. 199-211, doi: 10.1006 / jcat.1994.1336 .
- ↑ Brigitte Osterath: Process development for the production of 2-keto-L-gulonic acid, a vitamin C precursor. 2009, p. 6, urn : nbn: de: hbz: 5N-20434 .
- ↑ RD Hancock, R. Viola: Biotechnological approaches for L-ascorbic acid production. In: Trends in Biotechnology . 20 (7), 2002, pp. 299-305. PMID 12062975 .
literature
- J. Boudrant: Microbial processes for ascorbic acid biosynthesis: a review. In: Enzyme Microb Technol. 12 (5), 1990, pp. 322-329. PMID 1366548 , doi: 10.1016 / 0141-0229 (90) 90159-N .
- C. Bremus et al: The use of microorganisms in L-ascorbic acid production. In: J Biotechnol. 124 (1), 2006, pp. 196-205. PMID 16516325 , doi: 10.1016 / j.jbiotec.2006.01.010 .
Web links
- chemieunterricht.de: ascorbic acid
- ETH history of technology: Vitamin C synthesis ( Memento from February 19, 2012 in the Internet Archive )