3-hydroxy-2-butanone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 3-hydroxy-2-butanone | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 8 O 2 | |||||||||||||||||||||
| Brief description |
colorless, pleasantly smelling liquid, in dimeric form colorless to light yellow crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 88.11 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.01 g cm −3 (25 ° C) |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| boiling point |
147 ° C |
|||||||||||||||||||||
| Vapor pressure |
5 h Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
3-Hydroxy-2-butanone or acetoin is an organic compound that is formed by some bacteria as an intermediate product of the metabolism, but also occurs in parts of plants.
Isomerism
3-Hydroxy-2-butanone has a stereocenter, so there are two stereoisomers , ( R ) -3-hydroxy-2-butanone and ( S ) -3-hydroxy-2-butanone.
| 3-hydroxy-2-butanone | ||
| Surname | ( R ) -3-hydroxy-2-butanone | ( S ) -3-hydroxy-2-butanone |
| other names | ( R ) -acetoin | ( S ) -acetoin |
| Structural formula |

|
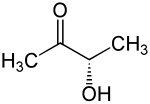
|
| CAS number | 53584-56-8 | 78183-56-9 |
| 513-86-0 (undefined) | ||
| EC number | 208-174-1 (undefined) | |
| ECHA info card | 100.007.432 (undefined) | |
| PubChem | 439314 | 447765 |
| 179 (undefined) | ||
| Wikidata | Q27098190 | Q59347812 |
| Q223083 (undefined) | ||
Occurrence
Known bacterial acetoin formers can be found, for example, in the genus Bacillus , among the enterobacteria and the lactic acid bacteria . Acetoin is produced during the anaerobic breakdown of glucose , but is only accumulated during certain fermentation processes ( 2,3-butanediol fermentation ). The ( R ) -isomer is formed here.
Under aerobic conditions, the compound is completely broken down into carbon dioxide (for example also in mammals).
The ability to form acetoin is used for rapid test procedures to determine unknown (intestinal) bacteria. The reaction with creatine and α-naphthol leads to the formation of a red dye in alkaline conditions ( Voges-Proskauer test , IMViC ).
Manufacturing
Acetoin can be obtained by reducing 2,3-butanedione or by enzymatic fermentation of carbohydrates with Bacillus tartaricus via acetaldehyde .
properties
Acetoin has a butter-like smell and is used to make flavors. It is also a natural component of apples , butter , yogurt , asparagus , black currants , blackberries , wheat , broccoli , Brussels sprouts and honeydew melons . Due to its α-hydroxyketone structure, it has a reducing effect, whereby it is oxidized to 2,3-butanedione ( diacetyl ).
use
The connection to the additives include, the cigarette manufacturers the tobacco add.
Individual evidence
- ↑ Entry on ACETOIN in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b entry on acetoin. In: Römpp Online . Georg Thieme Verlag, accessed on September 2, 2014.
- ↑ a b c d e f g h i Entry on acetoin in the GESTIS substance database of the IFA , accessed on February 13, 2017(JavaScript required) .
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, page 285, ISBN 978-3-906390-29-1 .
- ^ Siegfried Hauptmann : Organische Chemie , Verlag Harry Deutsch, 1st edition, Thun and Frankfurt am Main 1985, ISBN 3-87144-902-4 , p. 386.
- ↑ What's in a cigarette? (English) .

