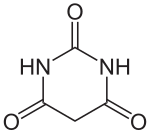
Barbituric acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Barbituric acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 4 N 2 O 3 | ||||||||||||||||||
| Brief description |
colorless, crystalline powder with a faint odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 128.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
250–252 ° C (decomposition) |
||||||||||||||||||
| pK s value |
4.01 (25 ° C) |
||||||||||||||||||
| solubility |
moderate in water (11.45 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−634.7 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Barbituric acid is a heterocyclic chemical compound that is used as a starting substance for the production of barbiturates and in analysis. The substance is one of the derivatives of urea , one of the lactams , is a cyclic ureide and at the same time a hydrogenated derivative of pyrimidine .
history
The constitution of barbituric acid was first recognized by Adolf von Baeyer in his studies on uric acid in 1864 . He is said to have named his discovery after his lover Barbara. According to other sources, the barbituric acid got its name in honor of the alchemist Barbara von Cilli (1390-1451).
Baeyer found that the compound can be hydrolyzed to form urea and malonic acid. The synthesis from these degradation products was carried out in 1879 by the French chemist Grimaux, who made them react with phosphorus oxychloride as a dehydrating agent.
Presentation and extraction
Barbituric acid can be prepared from urea and a malonic acid ester, e.g. B. Diethyl malonate , in anhydrous ethanol with sodium ethoxide dissolved in it:
properties
For barbituric acid, a tautomeric equilibrium can be formulated with a keto and an enol form . In the gas phase and in solution, the equilibrium is on the side of the keto form, which is the stable form under these conditions.
In the solid phase there are four polymorphic anhydrate forms and two polymorphic dihydrate forms . The crystal lattices of the anhydrate forms I, II and III contain the ketotautomer. Polymorph IV consists of molecules of the enol tautomer, which is the thermodynamically stable polymorph because of the stabilizing effect of hydrogen bonds . At 172 ° C. it shows an endothermic solid phase transition to polymorph II. Both polymorphs are enantiotropic to one another. The polymorph II transforms enantiotropically at 240 ° C into the high-temperature polymorph III, which then melts at 253 ° C. The dihydrate forms a monoclinic low-temperature form below -56 ° C. Above this temperature there is an orthorhombic high temperature form. Above 30 ° C this form dehydrates to anhydrate form II.
As the name suggests, the compound reacts sourly. Its p K s value is 4.01. Therefore, it was previously discussed whether the formula of barbituric acid should not be written as 2,4,6-trihydroxypyrimidine. This is a tautomeric form of the cyclic urea derivative.
It was later discovered that the imide form itself can function as an acid: its anion (barbiturate) is stabilized by delocalizing the negative charge.
use
Barbituric acid itself is not sedative- hypnotic. Only derivatives with suitable substituents have a hypnotic effect (see barbiturates ). The first such barbiturate to be described was diethylbarbituric acid ( barbital ) in 1903 by Emil Fischer and Joseph von Mering .
In drinking and wastewater analysis , barbituric acid is used as a reagent for colorimetric cyanide analysis (CN - ion).
Individual evidence
- ↑ a b c d e Entry on barbituric acid in the GESTIS substance database of the IFA , accessed on May 9, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-43.
- ↑ Entry on barbituric acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ^ Adolf Baeyer: Studies on the uric acid group. In: Justus Liebig's Annals of Chemistry. Volume 131, No. 3, 1864, pp. 291-302. doi: 10.1002 / jlac.18641310306 .
- ^ Werner E. Gerabek : Encyclopedia Medical History . Walter de Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 138 .
- ^ History of a sleeping pill. In: Pharmaceutical newspaper.
- ^ Grimaux: Bulletin de la Societe Chimique de France. Series 2, 31, 146, 1879. Annales de Chimie. Series 5, 17, 277. Quoted from Beilstein's Handbuch der Organischen Chemie. Volume 24, p. 467.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 468, ISBN 3-342-00280-8 .
- ^ F. Zuccarello, G. Buemi, C. Gandolfo, A. Contino: Barbituric and thiobarbituric acids: a conformational and spectroscopic study. In: Spectrochim. Acta . A 59, 2003, pp. 139-151, doi: 10.1016 / S1386-1425 (02) 00146-4 .
- ^ VB Delchev: DFT ab initio study of the keto-enol tautomerism of barbituric acid. In: J. Struct. Chem. 45, 2004, pp. 570-578, doi: 10.1007 / s10947-005-0031-8 .
- ↑ S. Ralhan, NK Ray: Density functional study of barbituric acid and its tautomers. In: J. Mol. Struct. THEOCHEM. 634, 2003, pp. 83-88, doi: 10.1016 / S0166-1280 (03) 00260-4 .
- ↑ M. Eigen, G. Ilgenfritz, W. Kruse: A kinetic method for investigating rapid prototropic tautomerization reactions. In: Chem. Ber. 98, 1965, pp. 1623-1638, doi: 10.1002 / cber.19650980539 .
- ↑ K. Senthilkumar, P. Kolandaivel: Quantum chemical studies on tautomerism of barbituric acid in gas phase and in solution. In: J. Comput.-Aided Mol. Des. 16, 2002, pp. 263-272, doi: 10.1023 / A: 1020273219651 .
- ↑ a b c d M. U. Schmidt, J. Brüning, J. Glinnemann, MW Hützler, P. Mörschel, SN Ivashevskaya, J. van de Streek, D. Braga, L. Maini, MR Chierotti, R. Gobetto: The thermodynamically stable Form of solid barbituric acid: the enol tautomer. In: Angew. Chem. 123, 2011, pp. 8070-8072, doi: 10.1002 / anie.201101040 .
- ↑ a b c G. S. Nichol, W. Clegg: A variable-temperature study of a phase transition in barbituric acid dihydrate. In: Acta Cryst. B 61, 2005, pp. 464-472, doi: 10.1107 / S0108768105017258 .
- ↑ a b N. Zencirci, E. Gstrein, C. Long, UJ Griesser: Temperature- and moisture-depent phase changes in crystal forms of barbituric acid. In: Thermochim. Acta . 485, 2009, pp. 33-42, doi: 10.1016 / j.tca.2008.12.001 .
- ↑ GA Jeffrey, S. Ghose, JO Warwicker: The Crystal Structure of Barbituric Acid Dihydrate. In: Acta Cryst. 14, 1961, pp. 881-887, doi: 10.1107 / S0365110X61002540 .
- ↑ E. Fischer, J. von Mering: About a new class of sleeping pills. In: Therapy of the Present. Volume 44 1903, pp. 97-101.
literature
- JR Partington: A History of Chemistry. Volume 4, 1964, OCLC 270846068 , p. 777.
- Hans Beyer, Wolfgang Walter: Textbook of organic chemistry. 21st edition. Hirzel, Stuttgart 1988, ISBN 3-7776-0438-0 , p. 784.


