Enols

Enols are chemical compounds of the general formula R 1 -CR 2 = CR 3 -OH. Its hydroxyl group is weakly acidic because the negative charge of the enolate is mesomerically stabilized . The simplest enol is the unstable ethenol , which immediately rearranges to acetaldehyde (ethanal) .
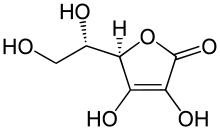
In the case of aliphatic enols, the equilibrium is completely on the keto side because of the energetic advantage, which is why enols only occur as derivatives - for example as phosphoenolpyruvate (PEP), the phosphoric acid ester of enolized pyruvic acid . However, aliphatic 1,3-dicarbonyl compounds are more enolized. For example, 76.4% of 2,4-pentanedione ( acetylacetone ) is present as cis -enol, which is stabilized via an intramolecular hydrogen bond . The cyclic enediol ascorbic acid is also almost entirely in the enol form.
In the case of phenols , the enol form is preferred over the keto form due to the formation of an aromatic system (see Hückel rule ).
See also
Individual evidence
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 377.