Phosphoenolpyruvic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Phosphoenolpyruvic acid | |||||||||||||||||||||
| other names |
2- (phosphonooxy) prop-2-enoic acid |
|||||||||||||||||||||
| Molecular formula | C 3 H 5 O 6 P | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 168.04 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Phosphoenolpyruvic acid ( PEP ), better known as its anionic form phosphoenolpyruvate , is a high-energy metabolic intermediate of glycolysis , of gluconeogenesis and the Hatch-Slack cycle of the C 4 plants . It also plays a role in some metabolic processes in humans, such as the transport of carbohydrates through biomembrane ( e.g. in the phosphotransferase system ), in the citric acid cycle and in glycolysis. It examines whether in the treatment of irritable bowel syndrome ( irritable bowel syndrome , IBS can be used) to relieve symptoms.
N - (phosphonomethyl) glycine, the active ingredient of glyphosate , works due to its chemical similarity to phosphoenolpyruvate (PEP), the regular substrate of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) by blocking the synthesis of the aromatic amino acids phenylalanine , Tryptophan and tyrosine via the shikimate pathway in plants, as well as in most microorganisms. These amino acids are essential for us humans, i. H. we have to take them in with our food, ultimately provided by microorganisms or plants.
The acid is produced in organisms by elimination of water with the catalytic action of the enolase from phosphoglyceric acid or phosphoglycerate, but can in principle also be produced by phosphorylation of the enol form of pyruvic acid with the supply of energy.
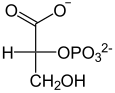 |
 |
ADP ATP pyruvate kinase |

|
||
| D - 2-phosphoglycerate | Phosphoenolpyruvate | Pyruvate |
2-phosphoglycerate splits off a water molecule; phosphoenolpyruvate (PEP) is formed. Due to the resulting double bond , the phosphate group of PEP is bound unstably and is easily transferred to ADP with formation of ATP ; pyruvic acid or pyruvate is produced from the PEP .
Three salts are known in crystalline form. Sodium pyruvate can be precipitated as a monohydrate from methanol and diethyl ether . The barium salt is known as hexahydrate and can be obtained by precipitation from an aqueous alcoholic solution. In addition, barium silver pyruvate was obtained as a dihydrate by precipitation from aqueous acetone .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Entry on phosphoenolpyruvate. In: Römpp Online . Georg Thieme Verlag, accessed on April 14, 2011.
![{\ mathrm {{\ xrightarrow [{Enolase}] {- Water}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f85bf9432f7fef6872de42b38cf4c6826038c435)