Shikimic acid route
| Parent |
| Metabolism of Carboxylic Acids |
| Gene Ontology |
|---|
| QuickGO |
The shikimic acid pathway or shikimate pathway is the name of a biochemical metabolic pathway that occurs in plants and most microorganisms . It is of fundamental importance through the biosynthesis of the proteinogenic aromatic amino acids phenylalanine , tyrosine and tryptophan . In addition, it provides important raw materials for the secondary plant metabolism .
Reaction sequence
Main path
By and large, shikimic acid runs away in a similar way in the various capable organisms, but there are differences in the details. It should therefore be pointed out that the following reaction scheme relates to the relatively well-researched shikimic acid pathway of the bacterium Escherichia coli .
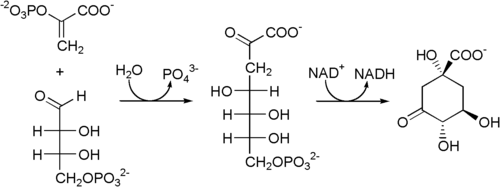
The reaction of phosphoenolpyruvate (PEP) and erythrose-4-phosphate, catalyzed by the enzyme 3-deoxyarabinoheptulosanate-7-phosphate synthase , is established as the starting point of the shikimic acid pathway . This creates 3-deoxyarabinoheptulosanate-7-phosphate, which is cyclized to 3-dehydroquinate in the next step by 3-dehydroquinate synthase .
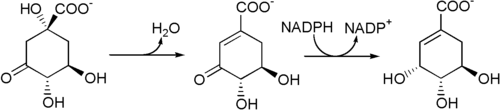
From 3-dehydroquinate, a dehydration mediated by the enzyme 3-dehydroquinate dehydratase results in 3-dehydroshikimate, which is then reduced to shikimate by the shikimate dehydrogenase .
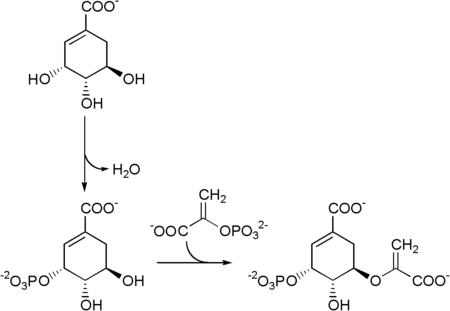
This is followed by the ATP- dependent phosphorylation of the shikimate by the shikimate kinase to form shikimate-3-phosphate . The reaction catalyzed by 5-enolpyruvylshikimate-3-phosphate synthase with a further molecule of phosphoenolpyruvate then gives 5-enolpyruvylshikimate-3-phosphate .
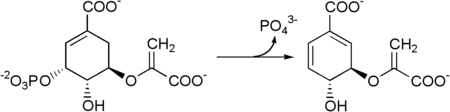
After the phosphate group has been split off by the chorismate synthase , it becomes chorismate .
The chorismate mutase catalyzes the conversion of chorismate into prephenate via a Claisen rearrangement .
Branches of the synthesis path


The synthesis pathways of the two amino acids tyrosine and phenylalanine only share with prephenate. For tyrosine synthesis, prephenate is converted into 4-hydroxyphenylpyruvate by a prephenate dehydrogenase . In the phenylalanine branch of the synthesis pathway, prephenate dehydratase catalyzes the reaction of prephenate to phenylpyruvate . In a final transamination step, the two precursors produce the respective amino acids tyrosine and phenylalanine. Alternatively, prephenate can also be transaminated to arogenate first, which is then either converted into phenylalanine by an arogenate dehydrogenase or into tyrosine by an arogenate dehydrogenase .
Even with the intermediate product chorismate, the synthetic route of the aromatic amino acid tryptophan branches off from the main path.

In the first step, the anthranilate synthase converts chorismate into anthranilate , which reacts with phosphoribosyl pyrophosphate to form N- (5-phosphoribosyl) anthranilate by means of the anthranilate phosphoribosyl transferase . An Amadori rearrangement catalyzed by phosphoribosylanthranilate isomerase leads to 1- (2-carboxyphenylamino) -1-deoxyribulose-5-phosphate. The indole-3-glycerol phosphate synthase produces the ring closure to indole-3-glycerol phosphate. The last two partial reactions, namely the splitting off of glyceraldehyde-3-phosphate to the intermediate product indole and its condensation with serine , catalyzes the tryptophan synthase , which supplies tryptophan.
Biological importance
As autotrophic organisms, plants dominate the shikimic acid pathway; it is part of their primary metabolism , but also provides precursors for secondary plant substances . Apart from the proteinogenic aromatic amino acids tryptophan , phenylalanine and tyrosine , an abundance of other substances with aromatic rings ultimately result from the shikimate pathway. Chlorogenic acid is also very important . a. is involved in herbal wound healing, although the following list is by no means exhaustive and the Shikimate path can also be combined with other synthetic pathways:
- Ubiquinones and plastoquinones
- Vitamin E , Vitamin K , Folic Acid
- Siderophores
- lignin
- Phenylpropanoids
- Isoflavones , anthocyanins , lignans , stilbenoids
- Cinnamic acid derivatives
- Coumarin , vanillin
- Benzoic acid derivatives ( salicylic acid , gallic acid , p-aminobenzoic acid )
- some alkaloids ( morphine , colchicine )
But the shikimic acid pathway has also been demonstrated in many bacteria , fungi and protozoa . In contrast, animals lack the appropriate enzymes. Therefore the amino acids phenylalanine and tryptophan are essential for them , tyrosine can only be synthesized directly from phenylalanine.
Since 2016 at the latest it has been known that many microbial strains also have the EPSPS enzyme (enolpyruvylshikimate-3-phosphate synthase) as a central substance in the Shikimate metabolism, an enzyme that destroys glyphosate when attacked on undesired plants. However, these microbes are also found in the intestines of young bees ; their destruction by glyphosate leads to a change in the intestinal flora of these animals, which then leads to an immunodeficiency . They stumble around disoriented and die.
In addition to the shikimic acid pathway, there are other options for the biosynthesis of aromatic ring structures, such as the polyketide pathway and nucleotide biosynthesis.
Others
- The compound glyphosate used in agriculture as a broad spectrum herbicide interrupts the shikimic acid pathway by inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EC 2.5.1.19).
- Since the presence of the shikimic acid pathway is common to many pathogens, the enzymes of this metabolic pathway are viewed as a promising point of attack in the development of drugs with a very broad spectrum of activity, which extends to bacterial , fungal and protozoal infections .
Individual evidence
- ↑ a b c d e f D. E. Metzler: Biochemistry. The Chemical Reactions of Living Cells. Volume 2. Elsevier Science, 2003; Pp. 1420-1471; ISBN 0-12-492541-3 .
- ↑ EC 2.5.1.54
- ↑ EC 4.2.3.4
- ↑ a b c d e f g h J. M. Berg, JL Tymoczko, L. Stryer: Biochemistry. 6th edition. Spectrum Academic Publishing House, Elsevier GmbH, Munich 2007; Pp. 773-775; ISBN 978-3-8274-1800-5 .
- ↑ EC 4.2.1.10
- ↑ EC 1.1.1.25
- ↑ EC 2.7.1.71
- ↑ EC 2.5.1.19
- ↑ EC 4.2.3.5
- ↑ EC 5.4.99.5
- ↑ EC 1.3.1.12 and EC 1.3.1.13
- ↑ EC 4.2.1.51
- ↑ EC 4.2.1.91
- ↑ EC 1.3.1.43 , EC 1.3.1.78 and EC 1.3.1.79
- ↑ EC 4.1.3.27
- ↑ EC 2.4.2.18
- ↑ EC 5.3.1.24
- ↑ EC 4.1.1.48
- ↑ EC 4.2.1.20
- ^ PM Dewick: Medicinal Natural Products: A Biosynthetic Approach. 3rd edition, John Wiley & Sons Ltd., 2009; Pp. 137-186; ISBN 978-0-470-74167-2 .
- ^ A b C. W. Roberts et al .: The shikimate pathway and its branches in apicomplexan parasites. In: J. Infect. Dis. 185 Suppl. 1, Feb 2002, pp. S25-36. PMID 11865437 .
- ↑ Pesticide changes microflora. Glyphosate and the immune deficiency of bees, by Joachim Müller-Jung , FAZ , September 25, 2018; on the microflora of the bee intestine