Phosphates
| Phosphates |
 The anion PO 4 3− |
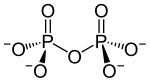 A condensate: diphosphate |
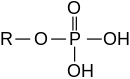 An ester: phosphoric acid ester |
Phosphates are the salts and esters of orthophosphoric acid (H 3 PO 4 ). In a broader sense, the condensates ( polymers ) of orthophosphoric acid and its esters are also called phosphates. The esters are described under phosphoric acid esters . Phosphorus is in the oxidation state (V) in all of these compounds . Oxygen compounds of phosphorus with other oxidation states are listed under phosphorus .
variants
Primary, secondary and tertiary phosphates
The salts of tri-basic ortho-phosphoric acid (H 3 PO 4 ) can be divided into primary, secondary and tertiary phosphates. In the case of monovalent cations M ', the empirical formulas apply according to M'H 2 PO 4 , M' 2 H 1 PO 4 and M ' 3 PO 4 . The partial neutralization of the phosphoric acid gives hydrogen or dihydrogen phosphates. These can react with both acids and bases . Because of this property, many buffer solutions contain hydrogen phosphates.
| primary phosphates (dihydrogen phosphates) |
secondary phosphates (hydrogen phosphates) |
tertiary phosphates |
|---|---|---|
| Sodium dihydrogen phosphate , NaH 2 PO 4 | Disodium hydrogen phosphate , Na 2 HPO 4 | Sodium phosphate , Na 3 PO 4 |
| Potassium dihydrogen phosphate , KH 2 PO 4 | Dipotassium hydrogen phosphate , K 2 HPO 4 | Potassium phosphate , K 3 PO 4 |
| Calcium dihydrogen phosphate , Ca (H 2 PO 4 ) 2 | Calcium hydrogen phosphate , CaHPO 4 | Calcium phosphate , Ca 3 (PO 4 ) 2 |
| For more examples see category: Phosphate | ||
Condensates
Phosphoric acid can form diphosphoric acid (H 4 P 2 O 7 ) in a condensation reaction (elimination of water) . Analogously there are corresponding salts, the diphosphates ( pyrophosphates ) M ' 4 P 2 O 7 . With continued reaction also form poly - or cyclo phosphates. Cyclo-phosphates are often called metaphosphates . Polyphosphates and metaphosphates are polymers of the salts of phosphoric acid.
| di-, poly- and cyclo-phosphates | ||
|---|---|---|
| Surname | reaction | Structure of the anion |
| di-phosphate (also: pyrophosphate) |

|
|
| tri-phosphate (gen .: poly-phosphate) |

|
|
| meta-phosphate (gen .: cyclo-phosphate) |

|
|
Pentasodium triphosphate (Na 5 P 3 O 10 ) and metaphosphates were used to soften water in detergents. As food additives z. B. Pentasodium triphosphate and diphosphates application.
Extraction
Phosphates are obtained from minerals such as apatite , Ca 5 [(PO 4 ) 3 (OH, F, Cl)]. The main deposits are in northern Africa ( Morocco , Western Sahara ), Jordan , the United States ( Florida ), Russia ( Kola Peninsula ), South Africa , Togo and China . In the past, the phosphate deposits with the highest concentration ( Nauruit , which originated from guano ) were found on the Pacific island of Nauru . The original deposits have been exhausted since 2003. In 2004 new deposits were developed on Nauru. Saudi Arabia has been one of the largest producers in the world since 2006.
The resources of phosphates is limited. Most are contaminated with cadmium and / or radioactive heavy metals. Some phosphate deposits have previously served as a source of uranium . It is assumed that the phosphate deposits that can be used for fertilizer production will be exhausted earlier than the global oil deposits . The cadmium content of the phosphate deposits varies greatly. Many industrialized countries have already introduced a limit value for cadmium in fertilizers. There is only one known deposit in the world that is below the EU limit (Kola). In developing countries, on the other hand, fertilization is carried out with cheaper cadmium-contaminated phosphate fertilizers .
The use of Thomas flour (a by-product of iron ore smelting), which was previously practiced in industrialized countries, is ruled out for health reasons due to the high chromium content. Another possibility is to use or recover the precipitated or biologically enriched phosphates present in the sewage sludge . In Germany and other countries, polluted sewage sludge is generally incinerated because it contains numerous heavy metals and endocrine disruptors .
Since 85% of the imported phosphate used in Germany goes into agriculture, some could be replaced by sewage sludge. According to the surveys of the Federal Statistical Office, fertilizer sales in the 2003/2004 financial year were 112,000 tons of phosphorus. This should be taken into account with the amendment of the Sewage Sludge Ordinance 2017, according to which "a recovery of phosphorus and a return of the recovered phosphorus or the phosphorus-containing sewage sludge incineration ash into the economic cycle should be aimed for". Corresponding considerations have also been made in Switzerland . The Swiss fertilizer ordinance was adapted accordingly in 2001 and a network for phosphorus recycling was set up in which other European countries are also involved. There are now seven corresponding pilot plants.
properties
Anions and pH values
Phosphate anions exist in three forms in aqueous solution . Under strongly basic conditions the phosphate anion is mainly present as (PO 4 3− ), while under weakly basic conditions the hydrogen phosphate anion (HPO 4 2− ) dominates. Under weakly acidic conditions, the dihydrogen phosphate anion (H 2 PO 4 - ) is mainly present. In strongly acidic aqueous solution, phosphoric acid (H 3 PO 4 ) is the main form.
So there are three pH-dependent equilibrium reactions:
| Equilibrium reactions | Equilibrium constant at 25 ° C | |
|---|---|---|
| (1) | ||
| (2) | ||
| (3) | ||
Under strongly alkaline conditions, such as B. at pH = 13 there is essentially PO 4 3− and HPO 4 2− . If the solution is neutral (pH = 7.0), H 2 PO 4 - (62%) and HPO 4 2− (38%) are present. At pH = 7.4 the ratio of the two components turns around: 39% H 2 PO 4 - and 61% HPO 4 2− . Under strongly acidic conditions (pH = 1), H 3 PO 4 is dominant compared to H 2 PO 4 - . HPO 4 2− and PO 4 3− are practically absent.
General
With the exception of the alkali and ammonium compounds, most phosphates are poorly soluble in water.
Phosphates can form compounds with heavy metals . This property makes the use of phosphates problematic, since the phosphates from the sewage sludge can mobilize heavy metals.
For the most part, deposits of phosphate compounds also contain heavy metals, such as B. Cadmium and Uranium .
Importance for nutrition
In the human diet, phosphate plays an essential role in energy metabolism and bone remodeling . It combines with calcium to form solid calcium apatite . The phosphate level is closely related to the calcium level. The importance of phosphate in the occurrence of hyperactivity in children has been disproved.
use
fertilizer
Most of the phosphates are used as fertilizer (see phosphate fertilizer , superphosphate , double superphosphate ). The suitability of phosphates for fertilization was discovered by chance: in iron and steel production according to the Thomas process , the phosphate-rich Thomas flour was a by-product , which turned out to be an excellent fertilizer.
As a result of erosion of agricultural land, phosphates bound to clay minerals find their way into rivers and lakes and from there into the seas. In limnic and marine ecosystems, they contribute significantly to eutrophication . Among other things, phosphates cause blue-green algae blooms ( cyanobacteria ) in the Baltic Sea.
Detergent additive
To soften water can pentasodium be used. In parts of Europe, phosphates are no longer used in detergents because they have led to over-fertilization and ultimately to the overturning of water bodies. Zeolite A is used as a substitute for this . However, tripolyphosphates are still used as softeners in machine dishwashing detergents. Tests by Stiftung Warentest in 2015 and 2016 showed that some phosphate-free dishwasher tablets already achieve a cleaning effect that is comparable to that of agents containing phosphate. The detergent regulation (EC) No. 648/2004 prescribes through the amending regulation (EU) No. 259/2012 in Annex VIa that from 1 January 2017 only machine dishwashing detergents for private consumers may be put on the market that are less than 0 , Contain 3 grams of phosphorus per standard dose.
Food additive
Sodium phosphate (E 339), potassium phosphate (E 340), calcium phosphate (E 341), magnesium phosphate (E 343) and the salts of orthophosphoric acid diphosphate (E 450), triphosphate (E 451) and polyphosphate (E 452) are food additives approved and used as preservatives , acidulants , acid regulators , release agents and emulsifiers . Phosphate is used for non-alcoholic, flavored drinks ( cola drinks ; in these also as phosphoric acid (E 338)), sterilized and ultra-high-temperature milk , concentrated milk, milk and skimmed milk powder and as a technical aid (prevents free-flowing foods from clumping together). Phosphates also play a very important role in food production (especially in the meat industry ) and are components of the melting salt for processed cheese .
Increased dietary phosphate intake increases blood pressure and pulse rate, even in healthy young adults.
Other uses
Animal feed , anti-corrosion agents (see phosphating ); Flame retardants ; Buffer substance for neutral pH range (see above), accumulators ( lithium iron phosphate accumulator ).
32 P, a radioactive isotope of phosphorus, is used in the form of dihydrogen phosphate (or sodium phosphate ) in a variety of ways in research and in nuclear medicine therapy, especially for polycythemia vera ( radiophosphorus therapy ).
proof
Detection reactions of phosphates are described under phosphorus .
Web links
Individual evidence
- ^ A b Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexikon der Chemie . Spectrum Academic Publishing House, Heidelberg, 2001.
- ^ Ma'aden Phosphate Development (Saudi Arabian Mining Company). Worley Parsons.
- ↑ Research report on phosphorus production from sewage sludge on behalf of the Federal Environment Agency , page 37
- ↑ see Sewage Sludge Ordinance § 3 Paragraph 1 Clause 2
- ↑ Federal Office for the Environment, FOEN 2009: Recovery of phosphorus from wastewater treatment
- ↑ News from the Swiss Phosphorus Network
- ↑ Manfred Döpfner: Attention Deficit / Hyperactivity Disorder (ADHD) . Hogrefe Verlag, 2013, ISBN 978-3-840-91939-8 ( limited preview in Google book search).
- ↑ Federal Institute for Risk Assessment : Hyperactivity and additives - is there a connection? BfR Opinion No. 040/2007 of September 13, 2007 . (PDF) .
- ↑ Phosphates in dishwashing detergents. IDW-Online, January 26, 2012.
- ↑ Dishwasher tabs tested by Stiftung Warentest In: test , 5/2015, pp. 62–66 and test.de from April 23, 2015.
- ↑ Dishwashing detergent: Now the phosphate-free ones can do it too! In: test , 8/2016, pp. 71–75 and test.de from October 5, 2016.
- ↑ EU: Report from the Commission to the European Parliament and the Council in accordance with Article 16 of Regulation (EC) No. 648/2004 of the European Parliament and of the Council of March 31, 2004 on detergents relating to the use of phosphates in consumer dishwasher detergents , May 29, 2015.
- ↑ EU: Regulation (EU) No. 259/2012 of the European Parliament and of the Council of March 14, 2012 amending Regulation (EC) No. 648/2004 with regard to the use of phosphates and other phosphorus compounds in intended for consumers Detergents and dishwasher detergents , March 30, 2012.
- ↑ Increased phosphate intake increases blood pressure in healthy adults. In: unibas.ch . August 23, 2018, accessed March 30, 2019 .
- ↑ Jaber Mohammad, Roberto Scanni, Lukas Bestmann, Henry N. Hulter, Reto Krapf: A Controlled Increase in Dietary Phosphate Elevates BP in Healthy Human Subjects. In: Journal of the American Society of Nephrology. 29, 2018, p. 2089, doi : 10.1681 / ASN.2017121254 .




![{\ displaystyle K_ {a1} = \ mathrm {\ frac {[H_ {2} PO_ {4} ^ {\, -}] [H_ {3} O ^ {+}]} {[H_ {3} PO_ { 4}]}} \ simeq 7 {,} 5 \ times 10 ^ {- 3}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/369a6c440b5efdda5a6819860cb4314e098e75a9)

![{\ displaystyle K_ {a2} = \ mathrm {\ frac {[HPO_ {4} ^ {2 -}] [H_ {3} O ^ {+}]} {[H_ {2} PO_ {4} ^ {\ , -}]}} \ simeq 6 {,} 2 \ times 10 ^ {- 8}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7f27bd3b099cf3f4c337951c1f6ba691be8c0748)

![K _ {{a3}} = {\ mathrm {{\ frac {[PO_ {4} ^ {{3 -}}] [H_ {3} O ^ {+}]} {[HPO_ {4} ^ {{2 -}}]}}}} \ simeq 2 {,} 14 \ times 10 ^ {{- 13}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/787b16a7960ba1083bae5e371cf83e4ed451a828)
