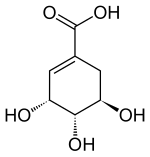
Shikimic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Shikimic acid | ||||||||||||||||||
| other names |
(3 R , 4 S , 5 R ) -3,4,5-trihydroxy-1-cyclohexenecarboxylic acid |
||||||||||||||||||
| Molecular formula | C 7 H 10 O 5 | ||||||||||||||||||
| Brief description |
light cream colored, odorless, needle-shaped crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 174.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
190-191 ° C |
||||||||||||||||||
| pK s value |
4.15 (14.1 ° C) |
||||||||||||||||||
| solubility |
180 g l −1 in water (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Shikimic acid is a biochemical intermediate in the metabolism of plants and microorganisms in the biosynthesis of aromatic amino acids. The natural product is (3 R , 4 S , 5 R ) -Shikimic acid. The other seven possible stereoisomers of shikimic acid have no biological significance.
history
Shikimic acid was first isolated in 1885 by Johan Fredrik Eykman from the Japanese star anise ( Illicium anisatum ), which is called Shikimi ( シ キ ミ , or in Kanji 樒 , 櫁 or 梻 ) in Japanese .
Extraction and presentation
Shikimic acid is an intermediate product in the biosynthesis of the aromatic amino acids L - tyrosine , L - tryptophan and L - phenylalanine in plants and microorganisms, which are essential for humans . Their salts are called shikimate.
Shikimic acid is a metabolic intermediate in plants and is therefore widespread. It occurs in large quantities in the poisonous shikimi fruit as well as in the related but non-toxic star anise .
Numerous aromatics found in plants are derived from shikimic acid, for example protocatechuic acid . Another route leads to gallic acid , which is part of the hydrolyzable tannins .
Shikimic acid route
The shikimic acid pathway is an important pathway for the biogenesis of aromatics. The shikimic acid pathway does not take place in higher animals.
The shikimic acid path doesn't actually begin with shikimic acid. Biosynthesis begins with the reaction of D - erythrose-4-phosphate with phosphoenolpyruvate (PEP). Only in further steps are dehydroquinic acid , dehydroshikimic acid, shikimic acid and chorismic acid formed . In the case of chorismate, the biosynthesis then divides into two paths. In one way L- tryptophan is produced, in the other via prephenate as an intermediate stage L- tyrosine and L- phenylalanine. The shikimic acid pathway is not only important for the protein structure in plants, starting from the α- amino acid formed in the shikimic acid pathway , but also for the biosynthesis of the coumarin derivatives and flavonoids .
application
Oseltamivir , the active ingredient in the flu drug Tamiflu ® , is produced from shikimic acid in a nine-step synthesis . The long synthetic route, which leads via dangerous azides , the low overall yield of around 35 percent and the complex extraction of the raw material shikimic acid from Chinese star anise currently make it difficult to produce oseltamivir in larger quantities. For some time now, however, shikimate can also be synthesized using recombinant E. coli strains.
Stereoisomers
Natural shikimic acid has the (3 R , 4 S , 5 R ) configuration. The other seven stereoisomers [(3 S , 4 R , 5 S ) -, (3 R , 4 R , 5 S ) -, (3 S , 4 S , 5 R ) -, (3 R , 4 S , 5 S ) -, (3 S , 4 R , R S ) -, (3 S , 4 S , 5 S ) - and the (3 R , 4 R , 5 R ) -Shikimic acid] have no practical significance. The racemate of (3 R , 4 S , 5 R ) - and (3 S , 4 R , 5 S ) -shikimic acid has a melting point of 191 to 192 ° C.
Individual evidence
- ↑ a b Data sheet (-) - Shikimic acid, 98% from AlfaAesar, accessed on June 19, 2019 ( PDF )(JavaScript required) .
- ↑ a b Entry on shikimic acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b The Merck Index , 9th edition, 1976, ISBN 0-911910-26-3 , p. 1097.
- ↑ a b Data sheet Shikimic acid, ≥99% from Sigma-Aldrich , accessed on June 19, 2019 ( PDF ).
- ^ Eykman, JF (1885). In: Recl. Trav. Chim. Pays-Bas . Vol. 4, p. 32.
- ↑ Jiang, S. and Singh, G. (1998): Chemical synthesis of shikimic acid and its analogues . In: Tetrahedron , Vol. 54, p. 4697. PDF ( Memento of the original from September 27, 2007 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Shikimic acid biosynthesis (English)
- ↑ Hoffmann-La Roche : Factsheet Tamiflu ( Memento of the original from February 22, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 336 kB), as of November 17, 2006.
- ↑ Tamiflu production secures Pharmaceutical newspaper issue 48/2005, accessed on September 25, 2018.
