Protocatechuic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Protocatechuic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 7 H 6 O 4 | |||||||||||||||||||||
| Brief description |
light brown solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 154.12 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
202–204 ° C (decomposition) |
|||||||||||||||||||||
| pK s value |
4.48 (COOH; 25 ° C) |
|||||||||||||||||||||
| solubility |
poor in water (20 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Protocatechuic acid (3,4-dihydroxybenzoic acid) is an aromatic compound that is derived from both benzoic acid and catechol (1,2-dihydroxybenzene). The structure consists of a benzene ring with an attached carboxy group (-COOH) and two hydroxyl groups (-OH) as substituents . It belongs to the group of dihydroxybenzoic acids , is contained in free form in the fruits of Japanese star anise ( Illicium religiosum ) and arises from the alkali melt of numerous natural substances , e.g. B. from catechins , from various resins, the dye Maklurin and several anthocyanins .
Occurrence
Protocatechuic acid is found in edible vegetables, fruits, and nuts, such as olives, brown rice, and pecans . It is also found in nettles .
presentation
Chemical synthesis
Protocatechuic acid is synthesized from p- hydroxybenzoic acid ; this is converted into m -chloro- p- hydroxybenzoic acid and the latter is heated with caustic potash under pressure.
The carboxylation with a Kolbe-Schmitt reaction by heating catechol with ammonium carbonate in a closed tube gives only poor yields, in aqueous solution no reaction takes place.
Another synthesis is based on vanillin and leads to protocatechuic acid via the demethylation of vanillic acid or its potassium salt:
When piperonlyic acid is heated with hydrochloric acid or hydriodic acid , protocatechuic acid is also formed. Equimolar amounts of formaldehyde are split off as a by-product .
biosynthesis
Protocatechualdehyde can be enzymatically oxidized to protocatechuic acid.
properties
Protocatechuic acid and melts at around 202–204 ° C and decomposes to catechol and carbon dioxide through decarboxylation . Your aqueous solution turns blue-green after the addition of ferric chloride . After adding a little soda or ammonia, this color first changes to purple, then to red. Protocatechuic acid has a strong reducing effect.
Reactions
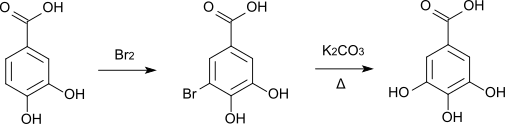
At room temperature, elemental bromine forms 5-bromoprotocatechuic acid , which is converted to gallic acid when it is melted with potassium carbonate .
If the bromination is carried out at 100 ° C., tetrabromo-catechol is formed with the elimination of carbon dioxide .
Physiological importance
The effect of protocatechuic acid on living cells is currently the subject of research and is still being discussed controversially. It has been shown to be a potent antioxidant with about 10 times more activity than α- tocopherol . In high doses, however, at 10 mM for example, it can also induce oxidative stress in cell cultures.
Compared to cancer cells protocatechuic shows different effects. It induces apoptosis in human gastric cancer cells (cells of a gastric adenocarcinoma ) and other tumor cells of the digestive tract and therefore acts as a cancer drug. However, there are also study data that indicate an opposite effect of protocatechuic acid. It induces cell division in neural stem cells and blocks apoptosis there. This has also been observed in mouse skin cancer cells. If there chemically by 12- O -Tetradecanoylphorbol 13-acetate cancer cells were induced protocatechuic could even increase tumor formation. When creating malignant cell lines of the human mandibular salivary gland in vitro , protocatechuic acid could not kill these cancer cells.
literature
- Paul Karrer : Textbook of Organic Chemistry . 10th edition. Georg Thieme, Stuttgart 1948, p. 569.
- German Chemical Society: Beilstein's Handbook of Organic Chemistry . Vol. 10, 4th edition, Springer, 1927: (system no. 1105) H 389 , EI 187 , EII 260
Individual evidence
- ↑ Entry on 3,4-DIHYDROXYBENZOIC ACID in the CosIng database of the EU Commission, accessed on May 13, 2020.
- ↑ a b c d Data sheet protocatechuic acid (PDF) from Merck , accessed on June 3, 2007.
- ^ D'Ans-Lax: paperback for chemists and physicists . 3rd edition, Volume 1, Springer, Berlin / Göttingen / Heidelberg 1967, ISBN 978-3-540-03756-9 . ( ChemieOnline - pK b and pK s values ).
- ↑ a b Data sheet 3,4-Dihydroxybenzoic acid from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ↑ a b Lin, HH., Chen, JH., Huang, CC., Wang, CJ .: Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK / p38 MAPK signaling activation. . In: Int J Cancer . 120, No. 11, June 2007, pp. 2306-2316. doi : 10.1002 / ijc.22571 . PMID 17304508 .
- ↑ Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection . In: Food Chemistry . tape 143 , January 15, 2014, p. 48-53 , doi : 10.1016 / j.foodchem.2013.07.097 .
- ↑ S. v. Kostanecki: On the introduction of the carboxyl group into the phenols. In: Reports of the German Chemical Society . 18, 1885, pp. 3202-3206 ( digitized on Gallica ).
- ↑ Irwin A. Pearl: Protocatechuic acid In: Organic Syntheses . 29, 1949, p. 85, doi : 10.15227 / orgsyn.029.0085 ; Coll. Vol. 3, 1955, p. 745 ( PDF ).
- ^ AH Parijs: The opening of the dihydroxymethylene ring In: Recueil des Travaux Chimiques des Pays-Bas . 1930, 49, No. 1. pp. 33-44, doi: 10.1002 / recl.19300490104
- ↑ R. Fittig, I. Remsen: Investigations on the constitution of piperine and its cleavage products piperic acid and piperidine . In: Justus Liebig's Annals of Chemistry . 168, No. 1, 1873, pp. 93-98, doi: 10.1002 / jlac.18731680110
- ↑ Georgios I. Panoutsopoulos, Christine Beedham: Enzymatic Oxidation of Vanillin, Isovanillin and Protocatechuic Aldehyde with Freshly Prepared Guinea Pig Liver Slices. In: Cell Physiol Biochem . 15, No. 1-4, 2005, pp. 89-98, PMID 15665519 ; ( PDF ).
- ^ A b J. Stenhouse: Action of Bromine on Protocatechuic Acid, Gallic Acid and Tannion. In: The chemical news . 29, 1874, p. 95 ( limited preview in Google book search).
- ^ Textbook of Chemistry full text
- ↑ a b Nakamura, Y. et al. : A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: dose-and timing-dependent enhancement and involvement of bioactivation by tyrosinase . In: Carcinogenesis . 21, No. 10, October 2000, pp. 1899-1907. doi : 10.1093 / carcin / 21.10.1899 . PMID 11023549 .
- ↑ a b H. Babich, A. Sedletcaia, B. Kenigsberg: In vitro cytotoxicity of protocatechuic acid to cultured human cells from oral tissue: involvement in oxidative stress . In: Pharmacol Toxicol . 91, No. 5, November 2002, pp. 245-253. doi : 10.1034 / j.1600-0773.2002.910505.x . PMID 12570031 .
- ↑ Guan, S. et al .: Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells . In: Toxicology in Vitro . 23, No. 2, March 2009, pp. 201-208. doi : 10.1016 / j.tiv.2008.11.008 . PMID 19095056 .
Web links
- Investigation of the antiviral activity (PDF file; 527 kB)