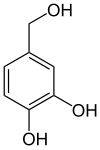
Protocatechual alcohol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Protocatechual alcohol | ||||||||||||||||||
| other names |
3,4-dihydroxybenzyl alcohol |
||||||||||||||||||
| Molecular formula | C 7 H 8 O 3 | ||||||||||||||||||
| Brief description |
white crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 140.14 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
135 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Protocatechual alcohol ( 3,4-dihydroxybenzyl alcohol ) is structurally derived from both benzyl alcohol and catechol .
It can be prepared from protocatechualdehyde in methanolic solution by reduction with hydrogen / Raney nickel at room temperature and normal pressure. It occurs naturally as a component of olive oil and in some living things.
Individual evidence
- ↑ a b c Hermann Kämmerer, Antonio Otzet Casacuberta: "For the representation of phenol alcohols from phenol aldehydes with hydrogen / Raney nickel and from phenol carboxylic acid esters with LiAlH 4 ", in: Die Makromolekulare Chemie , 1963 , 67 (1) , pp. 167-172 ; doi : 10.1002 / macp.1963.020670116 .
- ↑ a b Entry on 3,4-Dihydroxybenzyl Alcohol at TCI Europe, accessed on October 31, 2016.
- ↑ Marcello Saitta, Francesco Salvo, Giuseppa Di Bella, Giacomo Dugo, Giovanna Loredana La Torre: "Minor compounds in the phenolic fraction of virgin olive oils", in: Food Chemistry , 2009 , 112 (3) , pp. 525-532, doi : 10.1016 / j.foodchem.2008.06.001 .
- ↑ M. Sugumaran, V. Semensi, H. Dali, K. Nellaiappan: Oxidation of 3,4-dihydroxybenzyl alcohol: a sclerotizing precursor for cockroach ootheca , in: Archives of insect biochemistry and physiology , 1991 , 16 (1) , p 31-44.
