Benzyl alcohol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
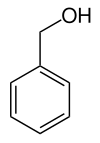
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Benzyl alcohol | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 7 H 8 O | ||||||||||||||||||
| Brief description |
colorless liquid with a slightly aromatic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 108.14 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.04 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−15 ° C |
||||||||||||||||||
| boiling point |
206 ° C |
||||||||||||||||||
| Vapor pressure |
0.07 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5396 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
not yet classified |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Benzyl alcohol or phenylmethanol is a chemical compound from the class of alcohols . It is used as a fragrance and aroma.
Occurrence and representation
Benzyl alcohol is about 6 percent in jasmine oil , but also in clove oil , gold lacquer oil , Peru balsam and Styrax included. It may by reacting benzyl chloride with alkali carbonates or by reduction of benzaldehyde are synthesized.
properties
Benzyl alcohol forms a colorless, somewhat oily liquid with a mild, pleasant scent but a bitter, numbing taste. The compound dissolves easily in most organic solvents, and it is also noticeably soluble in water. Benzyl alcohol is slowly oxidized to benzaldehyde by the oxygen in the air.
Esters of benzyl alcohol with various monocarboxylic acids have the character of a fragrance, such as benzyl acetate and benzyl benzoate .
use
It is an important solvent in the paint and ink industry, an additive for ballpoint pen pastes , dyeing auxiliaries and disinfectants , a development accelerator in color photochemistry and a starting product for the production of esters . It is also used as a preservative for injection solutions and for cosmetics.
It dissolves cellulose esters, cellulose ethers , fats , oils and resins , is an approved extraction solvent and reactive solvent in many construction chemicals and a component of paint strippers , many essential vegetable oils and is used as a viscosity regulator .
In the EU it is approved as a food additive with the number E 1519 for the production of flavors for certain beverages, confectionery and baked goods. A maximum quantity restriction of 5 mg per kilogram of body weight applies to benzoic acid and benzoates combined. Benzyl alcohol is considered harmless, but is known as an allergen .
Benzyl alcohol lotion for head lice
Benzyl alcohol is used in drugs against head lice ( Pediculus humanus capitis ). After contact with benzyl alcohol, the parasites can temporarily no longer close their respiratory openings. Oils can then clog the airways, killing the lice by suffocating.
Under the trade name Ulesfia ® , the US Food and Drug Administration (FDA) granted marketing approval in 2009 for an agent for the treatment of head lice, which contains 5% benzyl alcohol and 5% mineral oil as active ingredients. The lotion is applied to dry hair and rinsed out with water after an exposure time of ten minutes. The treatment must be repeated after 7 days as the product is not effective against nits . In a study with more than 600 subjects, 75% of the subjects were lousy-free after 14 days compared with 15% in the control group (active ingredient-free lotion). Skin and eye irritation may occur as side effects.
safety instructions
Benzyl alcohol is irritating to the skin and mucous membranes. It is harmful if swallowed and inhaled and causes serious eye irritation.
Benzyl alcohol was included in the EU's ongoing action plan ( CoRAP ) in 2015 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the consumption of benzyl alcohol were concerns about exposure of workers , high risk characterization ratio (RCR) and widespread use, as well as the possible hazard from sensitizing properties. The re-evaluation has been running since 2016 and is carried out by Germany . In order to be able to reach a final assessment, further information was requested.
Individual evidence
- ↑ Entry on BENZYL ALCOHOL in the CosIng database of the EU Commission, accessed on February 13, 2020.
- ↑ Entry on E 1519: Benzyl alcohol in the European database for food additives, accessed on August 6, 2020.
- ↑ a b c d e entry on benzyl alcohol. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e f g h Entry on benzyl alcohol in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-42.
- ↑ Entry on Benzyl alcohol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Juliane Daphi-Weber, Heike Raddatz, Rainer Müller: Investigation of Fragrances - Controlled Fragrances , pp. 94–95, in Volume V of the series HighChem hautnah - News from food chemistry (published by the Society of German Chemists ) 2010, ISBN 978- 3-936028-64-5 .
- ↑ FDA label for ULESFIA ( en , PDF, 93.3 kB) on the website of the Food and Drug Administration FDA . S. 5 April 9, 2009. Retrieved November 10, 2009.
- ↑ a b rme / aerzteblatt.de: Lotion suffocates head lice. (No longer available online.) In: aerzteblatt.de . February 24, 2010, archived from the original on September 20, 2015 ; accessed on October 11, 2015 .
- ^ Substance information Benzyl alcohol. ECHA, accessed on November 16, 2017 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): benzyl alcohol , accessed on March 26, 2019.
