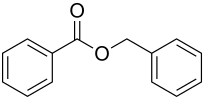
Benzoic acid benzyl ester
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Benzoic acid benzyl ester | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 14 H 12 O 2 | |||||||||||||||||||||
| Brief description |
colorless liquid with a slightly aromatic odor at temperatures above 20 ° C; needles or leaves below 20 ° C |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 212.24 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.12 g cm −3 |
|||||||||||||||||||||
| Melting point |
21 ° C |
|||||||||||||||||||||
| boiling point |
323 ° C |
|||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Benzoic acid benzyl ester is a chemical compound from the group of esters of benzoic acid .
Occurrence
Benzoic acid benzyl ester occurs naturally in some plants (e.g. balsam of Peru , balsam of tolu and the bark of the cinnamon tree ).
Extraction and presentation
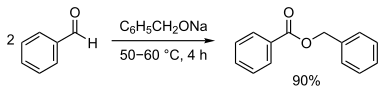
Benzoic acid benzyl ester can be obtained by a condensation reaction of benzoic acid and benzyl alcohol . It can also be prepared from benzaldehyde by a Claisen-Tiščenko reaction .
properties
Benzoic acid benzyl ester is rapidly hydrolyzed in the body to benzoic acid and benzyl alcohol . It has a viscosity of 10.9 mPa · s at 25 ° C. The flash point is 148 ° C, the ignition temperature is 480 ° C.
use
Benzoic acid benzyl ester is used as a synthetic chemical. It is also used in medicine against mites (e.g. for scabies ), in the perfume, cosmetics and food industries for disinfecting and preserving. In perfumery it is used as a solvent and fixative for fragrances, in photo technology as a camphor substitute in celluloid and in chemistry as a solvent (e.g. for cellulose derivatives). In the food industry it is used as an additive and additive to chewing gum flavors.
safety instructions
Benzyl benzoate has local irritant effects on the skin and mucous membranes.
Web links
- Comparative study by the WHO between benzyl benzoate and ivermectin in scabies (English) (PDF; 546 kB)
Individual evidence
- ↑ Entry on BENZYL BENZOATE in the CosIng database of the EU Commission, accessed on May 12, 2020.
- ↑ a b c d e f g h i j k l m Entry on benzyl benzoate in the GESTIS substance database of the IFA , accessed on December 22, 2019(JavaScript required) .
- ↑ a b c Benzyl benzoate (Allum)
- ↑ Entry on Benzyl benzoate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Eberhard Postel: Benzyl benzoate, a component of the Peru balsam , in: Klinische Wochenschrift , Volume 22, Numbers 20–21 / May 1943, pp. 362–364 ( doi : 10.1007 / BF01783698 ).
- ↑ Kamm, O .; Kamm, WF: Benzyl benzoate In: Organic Syntheses . 2, 1922, p. 5, doi : 10.15227 / orgsyn.002.0005 ; Coll. Vol. 1, 1941, p. 104 ( PDF ).
- ↑ Benzyl benzoate (embryotox) ( Memento from November 26, 2010 in the Internet Archive ).
- ↑ Benzoic acid benzyl ester data sheet (PDF) from Merck , accessed on March 14, 2010.
- ↑ Benzoic acid benzyl ester (benzyl benzoate) FCC ( Memento from November 7, 2017 in the Internet Archive ).
- ↑ entry to benzyl benzoate at Vetpharm, accessed on 22 November 2011th