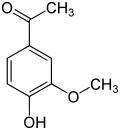
Vanillin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Vanillin | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 8 H 8 O 3 | ||||||||||||||||||
| Brief description |
colorless needles smelling of vanilla |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 152.14 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.06 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point | |||||||||||||||||||
| Vapor pressure |
0.29 Pa (25 ° C) |
||||||||||||||||||
| pK s value |
7.40 (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
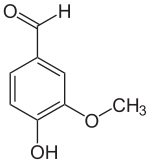
Vanillin ( 4-hydroxy-3-methoxybenzaldehyde , FEMA 3107 ) is the main flavoring substance in the capsule fruits of the spice vanilla ( Vanilla planifolia ) as well as a nature-identical flavoring substance . The organic chemical compound with the empirical formula C 8 H 8 O 3 is a derivative of benzaldehyde with an additional hydroxyl and methoxy group .
Vanillin is the main component of natural vanilla extract , a mixture of several hundred different compounds. Vanillin was isolated from this rather rare natural product as early as the middle of the 19th century, and in 1874 the first synthesis from the natural substance coniferin was achieved . The first commercial manufacturing processes for vanillin later came from eugenol . Today vanillin is as nature-identical cost of guaiacol synthesized or from lignin recovered, a constituent of wood and the most common byproduct of industrial pulp production . The vanillin in the lignin also contributes to the typical smell of old paper. In addition, several biotechnological processes have now been established whose products can be declared as "natural".
In terms of quantity, vanillin is the most important flavoring substance worldwide , and it can also be produced cheaply. It is used in food, beverages, ice cream, baked goods and chocolate, as well as in the perfume and pharmaceutical industries.
history
Vanilla was grown as a flavor by pre-Columbian peoples of Central America; at the time of their conquest by Hernán Cortés , the Aztecs used them as a flavoring for chocolate . Both chocolate and vanilla became known to Europeans around 1520.
Vanillin was first isolated as a relatively pure substance by Nicolas-Théodore Gobley in 1858 ; this was done by complete evaporation of a vanilla extract and subsequent recrystallization from hot water. In 1874, the chemist Wilhelm Haarmann, together with Ferdinand Tiemann, succeeded in producing vanillin from coniferin , which occurs in the bark sap of conifers , for the first time in Holzminden .

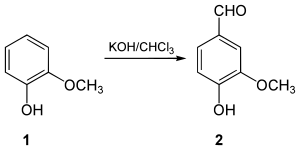
In 1876 Karl Reimer synthesized vanillin ( 2 ) for the first time from guaiacol ( 1 ). In the synthesis, later called the Reimer-Tiemann reaction , guaiacol is reacted with chloroform in an alkaline medium . First, chloroform reacts with the base to form dichlorocarbene . This settles the phenolate - anion to the guaiacol.
Occurrence
Vanillin is found most often in the capsule fruits of the spice vanilla ( Vanilla planifolia ) (1.5–4%), which are usually incorrectly referred to as pods , and also in styrax , cloves and other plants. The freshly harvested green seed pods contain vanillin in the form of its β- D -glucoside vanillosid . The green pods do not have the taste or smell of vanilla. Relatively pure vanillin can deposit as white dust or "frost" on the outside of the pods.
In lower concentrations, vanillin contributes to the taste and aroma of foods in a number of ways: in olive oil , butter, raspberries and lychee fruits. Vanillin also contributes to the flavor profile when wines and spirits are matured in oak barrels . In other foods, heat treatment creates vanillin from other existing ingredients. In this way, vanillin contributes to the taste and aroma of roast coffee , maple syrup and whole grains, including corn tortillas and oatmeal.
Extraction and presentation
Natural sources
The up to 30 cm long capsule fruits of the spiced vanilla are harvested shortly before ripening. These do not yet have the typical aroma and taste of the finished product. To obtain it, the fruits are subjected to what is known as black browning. First the capsule fruits are treated with hot water or steam, followed by fermentation in airtight containers. The drying and fermentation processes transform the β- D -glucosides of vanillin into vanillin and glucose .
A large part of the vanillin is obtained from the sulphite waste produced during pulp production . The lignosulphonic acid contained therein is treated with oxidants and alkalis at elevated temperature and pressure , whereby among other things vanillin is formed, which is purified by extraction , distillation and crystallization . The yields are 7–25% depending on the type of wood. This artificial vanilla flavor based on lignin has a richer taste profile. This is due to the presence of acetovanillon as a lignin by-product - an impurity that does not occur in vanillin from a guaiacol synthesis.
Technical syntheses
- Vanillin can be technically isomerization of eugenol ( 1 ) to isoeugenol ( 2 ) by means of alkalis, followed by oxidation by potassium permanganate or ozone gain.
- A laboratory-scale synthesis is carried out by electrophilic bromination of 4-hydroxybenzaldehyde ( 1 ) to 3-bromo-4-hydroxybenzaldehyde ( 2 ), followed by a copper-catalyzed methoxylation to vanillin ( 3 ):
- Using the Vilsmeyer-Haack synthesis , vanillin is obtained from guaiacol in about 70% yield. Guaiacol and N -methylformanilide react in the presence of phosphorus oxychloride as a catalyst to form vanillin.
- Guaiacol can be converted into vanillin and metanilic acid with formaldehyde and 3-nitrobenzenesulfonic acid in a process that takes several days .
- Another option is the substitution reaction of guaiacol ( 1 ) with glyoxylic acid and subsequent oxidation of the formed vanillylmandelic acid ( 2 ) to 4-hydroxy-3-methoxyphenylglyoxylsäure ( 3 ) to vanillin ( 4 ) is decarboxylated is.
- Another frequently used method is the Fries rearrangement of guaiacol acetate into acetovanillon and its subsequent degradation to vanillin.
Biotechnological methods
Alternatively, various biotechnological methods are available. Vanillin can be produced from ferulic acid, for example, by Amycolatopsis or Streptomyces strains . Ferulic acid can also be produced biotechnologically with the help of Pseudomonas strains from eugenol in a fed-batch process (eugenol is toxic to the cells). Eugenol is a readily available raw material and comes from clove oil. Curcumin also serves as a precursor to vanillin, with the help of the bacteria Rhodococcus rhodochrous this is obtained through biotransformation . It is also possible to obtain it from glucose using genetically modified Escherichia coli bacteria and subsequent dehydrogenase .
It can also be produced from yeast cultures via the shikimic acid route .
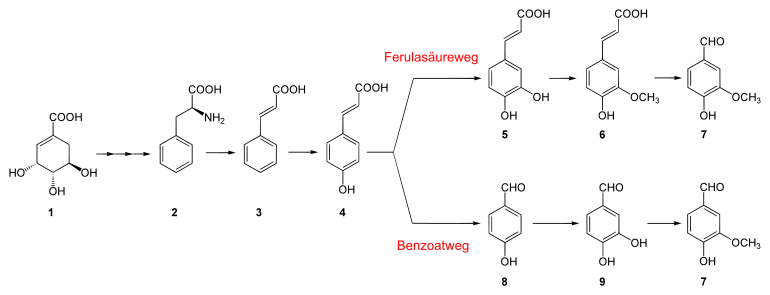
Vanillin as a product of the shikimic acid pathway ( 1 ). The end products of this path of chemical reactions are the amino acids phenylalanine , tyrosine and tryptophan . Phenylalanine ( 2 ) is biosynthetically converted to cinnamic acid ( 3 ) with the help of the enzyme phenylalanine ammonia lyase (PAL) with the release of ammonia (NH 3 ) . This is the first step in the biosynthesis of the phenylpropanoids .
Two main ways are under discussion how the steps to vanillin proceed on the basis of phenylpropanoid compounds : the ferulic acid route and the benzoate route . Both initially assume a p -hydroxylation of cinnamic acid to p -cumaric acid (4- hydroxycinnamic acid ) ( 4 ). This is followed by three reaction steps, the order of which is different, but ultimately leads to the target molecule.
- In the ferulic acid pathway , hydroxylation takes place at the 3-position in the ring to form caffeic acid ( 5 ) and then its methylation to ferulic acid ( 6 ), and finally the double bond is cleaved to form the aldehyde , vanillin ( 7 ).
- In the benzoate route , however, the double bond is first cleaved to form 4-hydroxybenzaldehyde ( 8 ), then hydroxylation at the 3-position in the ring to form protocatechualdehyde ( 9 ) and finally its methylation to vanillin ( 7 ).
The hydroxylations from 3 to 4 and from 8 to 9 are catalyzed by the enzyme diphenolase . In the latter reaction, diphenolase acts as monophenol oxidase ; this activity currently has a different EC number ( EC 1.14.18.1 ), but it is the same enzyme.
In contrast to chemical production (“nature-identical”), the biotechnologically produced vanillin can be declared as “natural”.
Biosynthetic vanillin is around 60 times more expensive than synthetic (2015) but still cheaper than natural.
properties
Physical Properties
Vanillin occurs in the form of colorless, characteristically sweet-smelling needles, which gradually oxidize to vanillic acid in moist air . It dissolves poorly in water (10 g / l at 25 ° C.), on the other hand, in ethanol and diethyl ether . The compound occurs in two polymorphic crystal forms. Form I melts at 82 ° C with a heat of fusion of 22.4 kJ mol −1 . It crystallizes in the monoclinic crystal system in the space group P 2 1 (space group no. 4) with the lattice parameters a = 1404.9 pm , b = 787.4 pm, c = 1501.7 pm, β = 115.45 ° and four Formula units per unit cell . Form II melts at 80 ° C with a heat of fusion of 20.7 kJ mol −1 . Both crystal forms are monotropic to one another, with Form I being the thermodynamically stable crystal form. Vanillin boils at 285 ° C at normal pressure in a CO 2 atmosphere or 154 ° C at negative pressure (13 hPa ).
Chemical properties
The substance is structurally derived from both benzaldehyde and guaiacol ( 2-methoxyphenol ). Due to its bifunctional character, vanillin is very reactive. A large number of derivatives can be synthesized by etherification , esterification or aldol condensation . Further reactions are possible by attacking the aromatic ring. A catalytic hydrogenation of vanillin leads to vanillyl alcohol or 2-methoxy-4-methylphenol . Vanillin can be enzymatically oxidized to vanillic acid. An aqueous solution of iron (III) chloride forms a blue-violet color with vanillin.
The pK s value of the phenolic OH group is 7.40 (25 ° C). At 9.99 this value is significantly lower than for phenol; the electron-withdrawing aldehyde group increases the OH acidity through its −M effect ; the phenolic OH bond is increasingly polarized. The pK s value of the 4-hydroxybenzaldehyde is moving at a similar value and amounts to 7.66; the lack of a methoxy group makes little difference here. For comparison, the guaiacol has ( 2-methoxyphenol ) with its pK s practical value of 9.98 no difference to the phenol with 9.99.
Isomers and structural relatives
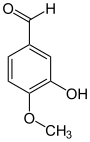
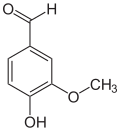
Isovanillin ( 3-hydroxy-4-methoxybenzaldehyde ) is an isomer and differs from vanillin in the position of the methoxy group. Instead of position 3, this is found here in position 4. Hydroxy and methoxy groups swap places compared to vanillin.
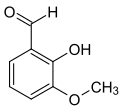
ortho- vanillin ( 2-hydroxy-3-methoxybenzaldehyde ) is also an isomer and differs from vanillin in the position of the hydroxyl group. The prefix ortho indicates the position of the hydroxyl group in the substitution pattern with respect to the aldehyde group; in vanillin these two groups are in para position.
 |
 |
|
| Isovanillin | Vanillin | ortho- vanillin |
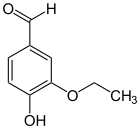
Ethylvanillin ( 3-ethoxy-4-hydroxybenzaldehyde ) is structurally related and differs from vanillin in that the methyl group is exchanged for an ethyl group. It does not occur naturally, but is produced by synthesis. Today it is often used as an artificial flavoring instead of the more expensive vanillin, as it costs about half as much and is two to four times more intense in taste and aroma.
Acetovanillon ( 4-hydroxy-3-methoxyacetophenone , also apocynin ) is also structurally related and differs from vanillin in that the aldehyde group is exchanged for an acetyl group . It is created in artificial vanilla flavors based on lignin .
 |
 |
|
| Ethyl vanillin | Vanillin | Acetovanillon |
Vanillin and ethylvanillin have a similar odor, but that of isovanillin is hardly noticeable. Vanillin and ethylvanillin can be easily separated using mixtures of hexane and ethyl acetate by thin-layer chromatography .
Veratrumaldehyde ( 3,4-dimethoxybenzaldehyde ), also methylvanillin, is also a structural relative with the same molecular formula.
Propenylguaethol (Vanitrope) is also used as a substitute; it has a typical vanilla aroma and its smell is about 15 times more intense than that of vanillin.
Analytics
The reliable qualitative and quantitative determination of vanillin in different test materials is possible after sufficient sample preparation by using gas chromatography or HPLC coupled with mass spectrometry .
The determination of vanillin can also be used to check the quality grades of olive oil as a marker substance. This analytical use to check authenticity in extremely fatty matrix, however, requires special procedures.
use
In terms of quantity, vanillin is the most important flavoring substance worldwide, not least because it can be manufactured at low cost. A consumption of around 15,000 tons per year is assumed (2004). The approximately 2,000 tons of capsule fruits of real vanilla , which are harvested worldwide every year, contain only about 40 tons of vanillin (the vanillin content of a commercial vanilla pod is between 1.6 and 2.4% according to ISO standard 5565-1: 1999). So over 99.7% of the vanillin placed on the market is not of natural origin.
Vanilla sugar is a preparation with the natural vanilla flavor of at least 1 g of ground vanilla pods or their extracts to 16 g of sugar ( sucrose ), vanilla sugar contains the flavoring addition of at least 0.17 g of vanillin to 16 g of sugar. Vanillin is used as a flavoring in various foods , including ice cream , baked goods, and chocolate . In addition, vanillin is one of many fragrances in perfume production and to improve the taste of pharmaceuticals and vitamin preparations , where it is used in small quantities to round off and fix sweet, balsamic scents.
Vanillin is also used in the chemical industry, for example as a starting material or intermediate in the synthesis of various medicinal products such as levodopa , methyldopa and papaverine . It is also part of Günzburg's reagent - an alcoholic solution of phloroglucin and vanillin for the qualitative detection of free hydrochloric acid in gastric juice .
Vanillin is used in histology in vanillin HCl staining to stain tannins . Vanillin can be used as a detection reagent for the derivatization of compounds in thin layer chromatography . The developed plate is wetted and heated by spraying or dipping with a vanillin-sulfuric acid solution. Some compounds show characteristic color reactions by means of which they can be identified.
More reactions and enzymes
- Vanillin dehydrogenase - enzyme that catalyzes vanillin to vanillic acid
- Vanillin Synthase Catalysis
- Vanillyl alcohol oxidase - enzyme that catalyzes various phenolic compounds through oxidation
- Vanillate monooxygenase - catalysis from vanillate to protocatechuic acid
literature
- Beilstein . E IV 8, 1763.
- German vanilla . In: The Gazebo . Issue 32, 1875, pp. 548 ( full text [ Wikisource ]).
- Alfons M. Burger: The natural and artificial flavors. Hüthig-Verlag, Heidelberg 1968.
- Jean-Paul Vidal: Vanillin. In: Food and Feed Technology. In: Kirk-Othmer Food and Feed Technology , Volume 2. John Wiley & Sons, New York 2007, ISBN 978-0-470-17448-7 , pp. 526-538, limited preview in Google Book Search, doi : 10.1002 /0471238961.2201140905191615.a01.pub2 .
- Andrew J. Taylor, Robert Linforth (eds.): Food Flavor Technology. Wiley-Blackwell, Oxford 2010, ISBN 978-1-4051-8543-1 , p. 170, limited preview in Google Book Search.
- Björn Bernhard Kuhse: Vanillin - history and school relevance. The history of a regional fragrance industry and its use in a practice-oriented chemistry class. Dissertation from Bielefeld University . Cuvillier Verlag, Göttingen 2010, ISBN 978-3-86955-459-4 , table of contents.
- Georg Schwedt : In the beginning there was vanillin: The fathers of the flavor industry in Holzminden. BoD, Norderstedt 2017, ISBN 978-3-7448-9306-0 , limited preview in the Google book search.
Web links
- chemgapedia (fragrances: vanillin / ethylvanillin as an example): Part 1 , Part 2 .
- Vanilla from orchids, vanillin from wood (www.chemieunterricht.de) .
- Simon Cotton (Uppingham School, Rutland, UK): Vanillin .
- 1 H-NMR spectrum at www.chemicalbook.com .
- Entry for vanillin in the Human Metabolome Database (HMDB) , accessed December 8, 2013.
Individual evidence
- ↑ a b Entry on FEMA 3107 in the database of the Flavor and Extract Manufacturers Association of the United States .
- ↑ Entry on VANILLIN in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d e f g h i j k l m entry on vanillin. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2014.
- ↑ a b c d e f g h i j Entry on vanillin in the GESTIS substance database of the IFA , accessed on February 22, 2014(JavaScript required) .
- ↑ a b c d e Braga, D .; Grepioni, F .; Maini, L .; Mazzeo, PP; Rubini, K .: Solvent-free preparation of co-crystals of phenazine and acridine with vanillin in Thermochim. Acta 507–508 (2010) 1–8, doi: 10.1016 / j.tca.2010.04.021
- ↑ Data sheet vanillin (PDF) from Carl Roth , accessed on August 5, 2016.
- ↑ a b c d e CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b c d Lawrence J. Esposito, K. Formanek, G. Kientz, F. Mauger, V. Maureaux, G. Robert, F. Truchet: Vanillin. In: Kirk-Othmer Encyclopedia of Chemical Technology. 4th edition. Vol. 24, John Wiley & Sons, New York 1997, pp. 812-825.
- ↑ N.-T. Gobley: Recherches sur le principe odorant de la vanille. In: Journal de Pharmacie et de Chimie . 34, 1858, pp. 401–405 ( limited preview in Google book search).
- ↑ Ferd. Tiemann, Wilh. Haarmann: About coniferin and its transformation into the aromatic principle of vanilla. In: Reports of the German Chemical Society . 7 (1), 1874, pp. 608-623 ( doi: 10.1002 / cber.187400701193 ).
- ↑ K. Reimer: About a new mode of formation of aromatic aldehydes. , In: Reports of the German Chemical Society . 9 (1), 1876, pp. 423-424 ( doi: 10.1002 / cber.187600901134 ).
- ^ W. Brandt, A. Braun, R. Brieger, H. Dieterle, R. Dietzel, W. Moeser, PN Schürhoff, F. Stadlmayr, O. Wiegand: Commentary on the German Pharmacopoeia. 6th edition. 1926, p. 661. (Reprint: Springer-Verlag, 2013, ISBN 978-3-642-90746-3 )
- ↑ Nicholas J. Walton, Melinda J. Mayer, Arjan Narbad: Vanillin. In: Phytochemistry . 63 (5), 2003, pp. 505-515 ( doi: 10.1016 / S0031-9422 (03) 00149-3 ).
- ↑ Manuel Brenes, Aranzazu García, Pedro García, José J. Rios, Antonio Garrido: Phenolic Compounds in Spanish Olive Oils. In: Journal of Agricultural and Food Chemistry . 47 (9), 1999, pp. 3535-3540 ( doi: 10.1021 / jf990009o ).
- ↑ Mohamed Adahchour, René JJ Vreuls, Arnold van der Heijden, Udo A. Th. Brinkman: Trace-level Determination of Polar Flavor Compounds in Butter by Solid-phase Extraction and Gas Chromatography-Mass Spectrometry. In: Journal of Chromatography A . 844 (1-2), 1999, pp. 295-305 ( doi: 10.1016 / S0021-9673 (99) 00351-9 ).
- ↑ Deborah D. Roberts, Terry E. Acree: Effects of Heating and Cream Addition on Fresh Raspberry Aroma Using a Retronasal Aroma Simulator and Gas Chromatography Olfactometry. In: Journal of Agricultural and Food Chemistry . 44 (12), 1996, pp. 3919-3925 ( doi: 10.1021 / jf950701t ).
- ^ Peter KC Ong, Terry E. Acree: Gas Chromatography / Olfactory Analysis of Lychee (Litchi chinesis Sonn.). In: Journal of Agricultural and Food Chemistry . 46 (6), 1998, pp. 2282-2286 ( doi: 10.1021 / jf9801318 ).
- ↑ Carole Viriot, Augustin Scalbert, Catherine Lapierre, Michel Moutounet: Ellagitannins and Lignins in Aging of Spirits in Oak Barrels. In: Journal of Agricultural and Food Chemistry . 41 (11), 1993, pp. 1872-1879 ( doi: 10.1021 / jf00035a013 ).
- ↑ Imre Blank, Alina Sen, Werner Grosch: Potent Odorants of the Roasted Powder and Brew of Arabica Coffee. In: Journal of Food Study and Research . 195 (3), 1992, pp. 239-245 ( doi: 10.1007 / BF01202802 ).
- ↑ P. Semmelroch, G. Laskawy, I. Blank, W. Grosch: Determination of Potent Odourants in Roasted Coffee by Stable Isotope Dilution Assays. In: Flavor and Fragrance Journal . 10 (1), 1994, pp. 1-7 ( doi: 10.1002 / ffj.2730100102 ).
- ↑ S. Kermasha, M. Goetghebeur, J. Dumont: Determination of Phenolic Compound Profiles in Maple Products by High-Performance Liquid Chromatography. In: Journal of Agricultural and Food Chemistry . 43 (3), 1995, pp. 708-716 ( doi: 10.1021 / jf00051a028 ).
- ↑ Ron G. Buttery, Louisa C. Ling: Volatile Flavor Components of Corn Tortillas and Related Products. In: Journal of Agricultural and Food Chemistry . 43 (7), 1995, pp. 1878-1882 ( doi: 10.1021 / jf00055a023 ).
- ↑ Helmut Guth, Werner Grosch: Odorants of Extrusion Products of Oat Meal. Changes During Storage. In: Journal of Food Study and Research . 196 (1), 1995, pp. 22-28 ( doi: 10.1007 / BF01192979 ).
- ↑ Martin B. Hocking: Vanillin: Synthetic Flavoring from Spent Sulfite Liquor. In: Journal of Chemical Education . 74 (9), 1997, pp. 1055-1059 ( doi: 10.1021 / ed074p1055 ).
- ^ A b Gary M. Lampman, Jennifer Andrews, Wayne Bratz, Otto Hanssen, Kenneth Kelley, Dana Perry, Anthony Ridgeway: The Preparation of Vanillin from Eugenol and Sawdust. In: Journal of Chemical Education . 54 (12), 1977, pp. 776-778 ( doi: 10.1021 / ed054p776 ).
- ↑ a b c Beyer / Walter : Textbook of organic chemistry . 19th edition. S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , p. 504.
- ↑ External identifiers or database links for 3-bromo-4-hydroxybenzaldehyde : CAS number: 2973-78-6, EC number: 608-409-1, ECHA InfoCard: 100.107.158 , PubChem : 76308 , Wikidata : Q63395282 .
- ↑ Douglass F. Taber, Shweta Patel, Travis M. Hambleton, Emma E. Winkel: Vanillin Synthesis from 4-Hydroxybenzaldehyde. In: Journal of Chemical Education . 84 (7), 2007, p. 1158 ( doi: 10.1021 / ed084p1158 ).
- ↑ N-Methylformanilide in the entry on Vanillin at ChemicalBook , accessed on August 5, 2016.
- ↑ Vanillin (PDF; 1.97 MB), accessed on July 31, 2016.
- ^ A b Bernd Schaefer: Natural Products in the Chemical Industry. Springer, 2014, ISBN 978-3-642-54460-6 , p. 116.
- ^ H. Prichert, J. Rabenhorst, A. Steinbüchel: Biotechnological Production of Vanillin. In: Applied Microbiology and Biotechnology . 56, 2001, pp. 296-314 ( doi: 10.1007 / s002530100687 ).
- ↑ Biotechnological production of vanillin .
- ↑ Suvendu Bhattacharya: Conventional and Advanced Food Processing Technologies. John Wiley & Sons, 2014, ISBN 978-1-118-40632-8 , p. 399.
- ↑ a b Oliver Kayser, Nils Averesch: Technical Biochemistry. Springer, 2015, ISBN 978-3-658-05547-9 , p. 86.
- ↑ Daphna Havkin-Frenkel, Andrzej Podstolki, Ewa Witkowska, Pjotr Molecki, Monika Mikolajczyk: Vanillin biosynthetic pathways: an overview. In: Tong-Jen Fu, Gurmeet Singh, Wayne R. Curtis: Plant Cell and Tissue Culture for the Production of Food Ingredients. American Chemical Society 1999. Division of Agricultural and Food Chemistry, pp. 35-43. ( limited preview in Google Book search)
- ↑ Patent WO03071861 : Vanillin Biosynthetic Pathway Enzyme from Vanilla planifolia. Published on September 4, 2003 , inventors: Daphna Havkin-Frenkel, Andrzej Podstolski, Richard A. Dixon.
- ↑ Osamu Negishi, Kenji Sugiura, Yukiko Negishi: Biosynthesis of Vanillin via Ferulic Acid in Vanilla planifolia. In: Journal of Agricultural and Food Chemistry . 57 (21), 2009, pp. 9956-9961 ( doi: 10.1021 / jf901204m . PMID 19817415 ).
- ↑ Peter Schopfer: Experimental Plant Physiology. Volume 2, Springer-Verlag, 2013, ISBN 978-3-642-61336-4 , p. 55.
- ↑ R. Dębowska, A. Podstolski: Properties of diphenolase from Vanilla planifolia (. Andr) Shoot primordia Cultured in Vitro. In: Journal of Agricultural and Food Chemistry . 49 (7), 2001, pp. 3432-3437 ( doi: 10.1021 / jf001180z . PMID 11453787 ).
- ↑ R. Velavan, P. Sureshkumar, K. Sivakumar, S. Natarajan: Vanillin-I. In: Acta Cryst. C51, 1995, pp. 1131-1133 ( doi: 10.1107 / S0108270194011923 ).
- ↑ Fragrance Lexicon: Vanillin .
- ↑ Georgios I. Panoutsopoulos, Christine Beedham: Enzymatic Oxidation of Vanillin, Isovanillin and Protocatechuic Aldehyde with Freshly Prepared Guinea Pig Liver Slices. In: Cell Physiol Biochem . 15 (1-4), 2005, pp. 89-98 ( PMID 15665519 ; (PDF)
- ↑ Entry on ethylvanillin. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ Toru Egawa, Akiyo Kameyama, Hiroshi Takeuchi: Structural Determination of Vanillin, Isovanillin and Ethylvanillin by Means of Gas Electron Diffraction and Theoretical Calculations. In: Journal of Molecular Structure . 794 (1–3), 2006, pp. 92–102 ( doi: 10.1016 / j.molstruc.2006.01.042 ; hokudai.ac.jp (PDF)
- ↑ AV Gerasimov, NV Gornova, NV Rudometova: Determination of Vanillin and Ethylvanillin in Vanilla Flavorings by Planar (Thin-Layer) Chromatography. In: Journal of Analytical Chemistry . 58 (7), 2003, pp. 677-684 ( doi: 10.1023 / A: 1024764205281 ).
- ^ PH List, L. Hörhammer: Hager's handbook of pharmaceutical practice. 7th volume: Dosage forms and auxiliary materials. Part B, 4th edition. Springer, 1977, ISBN 3-642-65823-7 , p. 37.
- ^ Y. Shen, B. Hu, X. Chen, Q. Miao, C. Wang, Z. Zhu, C. Han: Determination of four flavorings in infant formula by solid-phase extraction and gas chromatography-tandem mass spectrometry. In: J. Agric Food Chem . 62 (45), 2014, pp. 10881-10888. PMID 25338226 .
- ↑ J. Gerloff, IK Sundar, R. Freter, ER Sekera, AE Friedman, R. Robinson, T. Pagano, I. Rahman: Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. In: Appl. In Vitro Toxicol . 3 (1), 2017, pp. 28-40. PMID 28337465 .
- ↑ L. Franitza, M. Granvogl, P. Schieberle: Influence of the Production Process on the Key Aroma Compounds of Rum: From Molasses to the Spirit. In: J. Agric Food Chem . 64 (47), 2016, pp. 9041-9053. PMID 27788585 .
- ↑ DQ Li, ZQ Zhang, XL Yang, CH Zhou, JL Qi: Online restricted-access material combined with high-performance liquid chromatography and tandem mass spectrometry for the simultaneous determination of vanillin and its vanillic acid metabolite in human plasma. In: J. Sep. Sci . 39 (17), 2016, pp. 3318-3326. PMID 27384745 .
- ↑ M. Bononi, G. Quaglia, F. Tateo: Easy Extraction Method To Evaluate δ13C Vanillin by Liquid: Chromatography-Isotopic Ratio Mass Spectrometry in Chocolate Bars and Chocolate Snack Foods. In: J. Agric Food Chem . 63 (19), 2015, pp. 4777-4781. PMID 25965784 .
- ↑ NP Kalogiouri, NA Alygizakis, R. Aalizadeh, NS Thomaidis: Olive oil authenticity studies by target and nontarget LC-QTOF-MS combined with advanced chemometric techniques. In: Anal. Bioanal. Chem. 408 (28), 2016, pp. 7955-7970. PMID 27585916 .
- ↑ Guidelines for Vanilla Sugar and Vanilla Sugar (published by the Federation for Food Law and Food Science 1962), in which the general understanding of the food industry is laid down; New edition 2007.