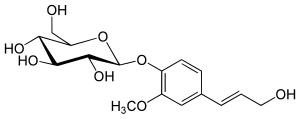
Coniferin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Coniferin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 22 O 8 | ||||||||||||||||||
| Brief description |
colorless needles with a slightly bitter taste |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 342.35 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
186 ° C (as dihydrate) |
||||||||||||||||||
| solubility |
Slightly soluble in cold water (5 g · l −1 ), good in boiling water, insoluble in ether |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Coniferin is a glucoside of coniferyl alcohol , that in the juice of the nascent formation in young wood of coniferous woods ( conifers ) occurs.
Occurrence
Theodor Hartig was first able to characterize it in 1861 in the cambial sap of the larch. The pharmacist Wilhelm Kubel from Holzminden first identified the glucoside of coniferyl alcohol in 1866. Coniferin is the main glycoside of conifers and was z. B. from the larch Larix decidua , the spruce Picea abies , the pines Pinus cembra and Pinus strobus and from the ash Fraxinus quadrangulata isolated. But it is also found in asparagus , salsify , sugar beet and other plants.
Extraction
Coniferin is obtained when freshly felled coniferous logs are debarked at the time of wood formation (in spring and at the beginning of summer) , the cambial juice is collected by scraping off the wood that is being formed, boiled, filtered , evaporated and the separated crystals are cleaned by recrystallization .
properties
In coniferin, D- glucose is β1-glycosidically linked to coniferyl alcohol. As a dihydrate, it forms colorless needles, is soluble in water and alcohol, but not in ether . Coniferin tastes slightly bitter, is odorless and weathers in the air.
Coniferin is split into D - glucose and coniferyl alcohol after heating with dilute acids or by the enzyme emulsin (a β- glucosidase) . Coniferin turns an intense blue when moistened with phenol and concentrated hydrochloric acid . This detection reaction can also be used to find coniferin in the various softwoods. Coniferin can be oxidized to vanillin with potassium dichromate and sulfuric acid . Coniferin was initially used to produce vanillin, which could soon be obtained more efficiently from eugenol .
biochemistry
Coniferin is the storage and transport form of coniferyl alcohol, which is used for the biosynthesis of lignin and numerous phytoalexins .
Individual evidence
- ↑ a b c d e f g Tiemann, F. & Haarmann, W. (1874): About coniferin and its transformation into the aromatic principle of vanilla. In: Reports of the German Chemical Society. Vol. 7, pp. 608-623. doi : 10.1002 / cber.187400701193 .
- ↑ a b c d Entry on coniferin. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 420, ISBN 978-0-911910-00-1 .
- ↑ There is not yet a harmonized classification for this substance . A label of [No public or meaningful name is available] in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 19, 2019, is derived from a self-classification by the distributor .
- ↑ W. Kubel, Coniferin - a glucoside from the cambial juice of the conifers in Journal für Praktische Chemie 97 , 243-246 (1866).
- ↑ Hermann Ammon (Ed.): Hunnius Pharmaceutical Dictionary . 8th edition. de Gruyter, Berlin 2004, ISBN 3-11-015792-6 .
Web links
- Coniferin . In: Meyers Konversations-Lexikon . 4th edition. Volume 4, Verlag des Bibliographisches Institut, Leipzig / Vienna 1885–1892, p. 247. - Basis for this article

