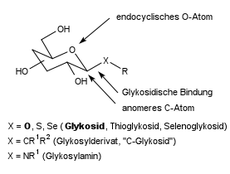
Glycosidic bond
A glycosidic bond or glycosidic bond is the chemical bond between the anomeric carbon atom of a carbohydrate ( glycon ) and the hetero or rarely carbon atom of an aglycone or a second sugar molecule. Compounds that contain a glycosidic bond are common in nature and are called glycosides .
If the aglycone is alcohol or phenol , then the bridging oxygen atom comes from the aglycone. Glycosides can also have bonds to other heteroatoms such as sulfur , selenium , nitrogen and phosphorus or rarely to carbon ("C-glycosides"). The glycosidic bond is hydrolytically cleavable, the reaction equilibrium being on the part of the cleavage products. The bond is kinetic but very stable. It is formed by a condensation reaction with little energy expenditure, with elimination of water . In nature, as is done glycosylation designated formation enzymatically via an activated saccharide in the laboratory by specific activation methods or by reaction of a sugar with a large excess of the alcohol under acid catalysis.
In the case of a glycoside, the aldehyde function of the aldoses (e.g. glucose ) or the keto function of the ketoses (e.g. fructose ) is present as a cyclic full acetal. An acetal is the condensation product of an aldehyde or ketone and one or two alcohols (“ hemiacetal ” or “full acetal”). Full acetals are stable to basic and neutral to weakly acidic aqueous solutions, but hydrolyze in the presence of strong acids .
Nomenclature and Examples
Simple glycosides

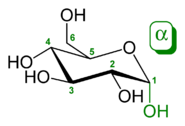
The position of the hydroxyl group of the anomeric carbon atom involved in the bond determines the name of the compound and also the type of bond: Glucose occurs in the cyclic pyranose form as α- D- glucopyranose or β- D- glucopyranose . This is taken into account in the nomenclature of a bond as an α- or β-glycosidic bond . In the case of a simple glycoside, for example made from the monosaccharide glucose and an alcohol such as ethanol , this is referred to as either ethyl-α- D -glucopyranoside or ethyl-β- D -glucopyranoside . While a glycosidic bond is normally a CO bond, in the case of glycosides with a heteroatom (S, Se, N, P) this is taken into account by mentioning it in the name, e.g. B. Ethyl S- α- D -glucopyranoside.
Definition of α and β
The anomeric configuration is defined by the prefixes α and β . The decisive factor is the stereochemical relation of the exocyclic oxygen to the configuration-determining carbon atom of the sugar (α = trans, β = cis). Here, α is not always axial and β is not always equatorial to the ring. Examples: In α - D -glucopyranose, the exocyclic oxygen is cis to the configuration-determining C-5: the aglycon is axial (here β is equatorial). In the prime example β - D - or L -arabinopyranose, the exocyclic oxygen is cis to the configuration-determining C-4. So here β is axial . The distinction is best seen in the Fischer spelling .
Di-, oligo- and polysaccharides
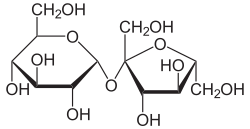


When two carbohydrates are condensed to form a disaccharide via a glycosidic bond, the nomenclature of the bond takes two different factors into account:
- On the one hand, depending on the starting compound, the α or β position of the first anomeric carbon atom involved in the bond.
- The second part of the name comes from the position of the carbon atoms that are directly bound to the oxygen bridge. In the case of sucrose (cane sugar), the bond is established via the 1st carbon atom of glucose with a hydroxyl group in the α position to the 2nd carbon atom of β-D-fructose. The bond is therefore an α-1 → 2-glycosidic bond and sucrose is therefore correctly called 1-α- D- glucopyranosyl-2-β- D -fructofuranoside or 2-β- D- fructofuranosyl-1-α- D -glucopyranoside .
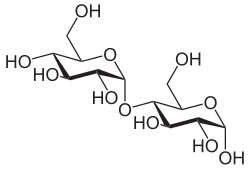
It is also possible that two identical monosaccharides are combined to form a disaccharide like cellobiose . The oxygen atom bridges the C1 atom of the first β- D -glucose molecule with the fourth carbon atom of a second, identical monomer. The compound is a β-1 → 4-glycosidic bond and the cellobiose is therefore the 1-β- D -glucopyranosyl-4-β- D -glucopyranoside. Since the anomeric first carbon atom of the second glucose molecule remains free, it can react with other monomers to form a polysaccharide such as cellulose. Analogously, two glucose molecules can be linked α-1 → 4-glycosidically to form maltose (1-α-D-glucopyranosyl-4-α-D-glucopyranose).
Another connection option occurs in gentiobiosis : two glucose units are 6-bridged via the 1st carbon atom of the first monomer and the 6th carbon atom of the second β-1 → 6-bridged, the connection is also called 1-β-D-glucopyranosyl -6-β-D-glucopyranose. In laminaribiosis , two glucose molecules are also β-1 → 3-linked.
Various glycosidic bonds occur in polysaccharides:
- Amylopectin , the main component of starch , is made up of α-1 → 4-linked glucose monomers.
- Amylose , which is also found in starch, only contains α-1 → 4-bridged glucose, which forms a helix structure.
- Cellulose consists of glucose, which is β-1 → 4-linked.
- Glycogen consists of glucose, with the main chain predominantly showing α-1 → 4 bonds, but also branches with an α-1 → 6 structure.
Complex glycosides and heteroglycosides


In addition to simple alcohols and other saccharides, the sugar content can also be linked to a more complex alcohol (such as a steroid alcohol), a phenol or another heteroatom such as nitrogen , sulfur or phosphorus . This case occurs, for example, in the nucleosides and nucleotides in which there is an N-glycosidic bond via a nitrogen bridge. The sugar content is mostly ribose or deoxyribose . In nature there are also a lot of O- and N- glycosides, more rarely S- glycosides, which have a wide variety of functions such as flavoring substances ( amygdalin , glycyrrhizin ), defense substances ( mustard oil glycosides , barbaloin , cycasin ), energy stores and carriers ( ADP , ATP ), Dyes ( azalein , betanine , carmine , malvin ) and storage substances ( coniferine ) meet. Some natural and artificial antibiotics such as azithromycin , bleomycin , cethromycin , doramectin and streptomycin are also glycosides.
Individual evidence
- ↑ Wissenschaft-Online-Lexika: Entry on glycosides in the compact lexicon of biology , accessed on February 25, 2009.
- ↑ a b IUPAC Nomenclature of Carbohydrates or Section 2-Carb-33: Glycosides and glycosyl compounds .
literature
- G. Löffler, PE Petrides, P. C: Heinrich: Biochemie & Pathobiochemie. 8th edition, Springer, Heidelberg 2006, ISBN 978-3-540-32680-9 .
- HR Horton, LA Moran, KG Scrimgeour, MD Perry, C. Biele, JD Rawn: Biochemistry. , 4th Edition, Pearson Education, 2008, ISBN 978-3-8273-7312-0 , 1088 pages.