Ribose
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Fischer projection , open-chain representation | ||||||||||
| General | ||||||||||
| Surname | Ribose | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 5 H 10 O 5 | |||||||||
| Brief description |
colorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 150.13 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
0.80 g cm −3 (20 ° C) |
|||||||||
| Melting point |
90-95 ° C |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Ribose is a sugar with five carbon atoms, a pentose , and occurs frequently in nature as D- ribose, while the enantiomeric L- ribose is of little importance.
D- ribose is part of the building blocks of ribonucleic acid (RNA). In the nucleosides , the ribose is linked to a nucleobase via the C1 atom , for example in adenosine , cytidine , guanosine , uridine and ribothymidine . The corresponding nucleotides are formed by additional phosphorylation of the hydroxyl group (-OH) on the C5 atom . The backbone of an RNA macromolecule is formed by the riboses linked to one another via phosphoric acid ester bonds.
“Ribose” usually means D- ribose. D - deoxyribose in deoxyribonucleic acid (DNA) only differs from this at the C2 atom due to a missing oxygen atom . Ribose can also be synthesized in the human organism from other monosaccharides via the pentose phosphate cycle .
properties
Ribose is a colorless solid with a melting point of 90–95 ° C and is soluble in water.
Ribose forms a hemiacetal in aqueous solution and can be present as a five-membered ring of a furanose or as a six- membered ring of a pyranose . In both cases, α- and β- anomeric forms are possible.
At 31 ° C, 58.5% of the D- ribose molecule is in the β- D -pyranoids, 21.5% in the α- D -pyranoids, and 13.5% in the β- D -furanoids, 6.5% in the α- D furanoid and 0.05% in the open-chain form. The β-pyranose form is therefore the most common in aqueous solution, since three of the four hydroxyl groups are located in the equatorial plane.
| D -Ribose spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
 α- D ribofuranose |
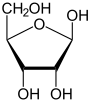 β- D- ribofuranose |
 α- D- ribopyranose |
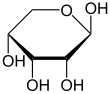 β- D- ribopyranose |
|
Biological importance

Ribose is a building block of nucleosides and nucleotides . Ribosyl nucleoside phosphates play an important role in the metabolism of cells, for example as biological energy carriers such as adenosine diphosphate (ADP) and adenosine triphosphate (ATP). The effect of hormonal and nervous signals in the cell can be enhanced by cyclic adenosine monophosphate (cAMP), which serves as a secondary messenger substance .
proof
Ribose can be detected with the Bial reagent (a solution of orcin and iron (III) chloride in concentrated hydrochloric acid ). The test is positive if a green-blue color changes after adding Bial reagent to the carbohydrate and after heating.
Web links
Individual evidence
- ↑ a b c d e f Entry for CAS no. 50-69-1 in the GESTIS substance database of the IFA , accessed on September 11, 2014(JavaScript required) .
- ↑ Stephen J. Angyal: The Composition of Reducing Sugars in Solution. In: Advances in Carbohydrate Chemistry and Biochemistry . Volume 42, 1984, pp. 15-68, doi : 10.1016 / S0065-2318 (08) 60122-5 .