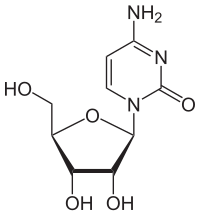
Cytidine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Cytidine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 9 H 13 N 3 O 5 | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 243.22 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
210–220 ° C ( decomposition ) |
|||||||||||||||||||||
| solubility |
soluble in water (50 g l −1 ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Cytidine is a nucleoside and consists of the nucleobase cytosine and the sugar β- D- ribose . The analogue with deoxyribose is deoxycytidine .
properties
Cytidine is part of ribonucleic acid (RNA) and forms a base pair there with guanosine .
Cytidine is an intermediate of the pyrimidine - metabolism , from the by cytidine deaminase ( EC 3.5.4.5 ) catalyzed deamination uridine , uridine by cytidine kinase ( EC 2.7.1.48 ) catalyzed phosphorylation of cytidine 5'-monophosphate (CMP) is formed. The latter is further phosphorylated via cytidine-5'-diphosphate (CDP) to cytidine-5'-triphosphate (CTP). CDP or CTP serve u. a. as building blocks in ribonucleic acid (RNA) synthesis or as an activating group in the synthesis of lipids such as lecithin , cephalin and cardiolipin .
The breakdown to cytosine takes place through the catalytic activity of pyrimidine nucleosidase ( EC 3.2.2.8 ).
Related links
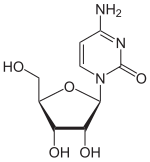
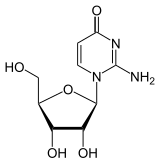
Isomers
|
|
| Cytidine, C. | Isocytidine , iC |

|

|
| Pseudocytidine , ψC | Pseudoisocytidine , psiC |
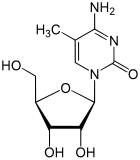
Methylated derivatives

|
|

|

|
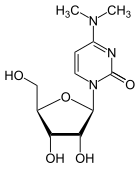
|
| 3-methylcytidine , m 3 C | 5-methylcytidine , m 5 C | 2'-O-methylcytidine , C m | N 4 -methylcytidine , m 4 C | N 4 , N 4 -dimethylcytidine , m 4 2 C |
Aza derivative

|
| 5-azacytidine , 5-azaC |
Web links
- Entry for Cytidine in the Human Metabolome Database (HMDB) , accessed October 12, 2013.
- Modification Summary of Cytidine in the Modomics database, accessed January 14, 2014.
Individual evidence
- ↑ a b c d e f data sheet Cytidine, BioReagent, suitable for cell culture, powder, ≥99% from Sigma-Aldrich , accessed on December 21, 2019 ( PDF ).
- ↑ Gerhard Michal (Ed.): Biochemical Pathways - Biochemie Atlas , Spektrum, Akad. Verl., Heidelberg 1999, ISBN 3-86025-239-9 .
