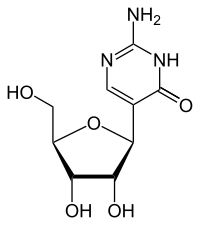
Pseudoisocytidine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| Pseudoisocytidine (3 H tautomer) | |||||||||||||
| General | |||||||||||||
| Surname | Pseudoisocytidine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 9 H 13 N 3 O 5 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 243.22 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Pseudoisocytidine is a synthetic nucleoside . It consists of the sugar β- D- ribofuranose and the nucleobase isocytosine . Pseudoisocytidine is an isomer of cytidine . Other isomers are isocytidine and pseudocytidine .
properties
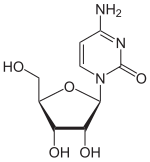
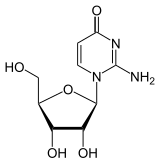
Chemically, it is a C- glycoside in which the β- D - ribose is linked to the C 5 -atom of the base isocytosine. In contrast, in cytidine and isocytidine the ribose is linked to an N atom. This type of linkage between ribose and base is similar to that in pseudouridine . Due to the isomerism of cytidine to isocytidine, the amino group and carbonyl group swap places. Due to the isomerism to pseudocytidine, another exchange takes place, so that the substitution pattern of the cytidine is found again.
|
|

|

|
| Cytidine, C. | Isocytidine, iC | Pseudoisocytidine, psiC (1 H -automer) |
Pseudoisocytidine, psiC (3 H tautomer) |
Pseudoisocytidine occurs in two tautomers , the proton is either on the N 1 or on the N 3 nitrogen.
use
Pseudoisocytidine is considered to be a more stable analogue of 5-azacytidine , especially against various 1-β- D -arabinofuranosylcytosine -resistant strains of leukemia in mice.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Lou-Sing Kana, WC. Lina, R. Dayal Yadava, JH Shiha & Ito Chaoa: "NMR Studies of the Tautomerism in Pseudoisocytidine", Nucleosides and Nucleotides , 1999 , 18 (4-5), pp. 1091-1093 ( doi : 10.1080 / 15257779908041655 ).
- ↑ JH Burchenal, K. Ciovacco, K. Kalaher, T. O'Toole, R. Kiefner, MD Dowling, CK Chu, KA Watanabe, I. Wempen, JJ Fox: "Antileukemic Effects of Pseudoisocytidine, a New Synthetic Pyrimidine C- Nucleoside ", Cancer Research , 1976 , 36 (4), pp. 1520-1523 ( PMID 1260769 ; PDF ).