from Wikipedia, the free encyclopedia
Structural formula
General
Surname
Isocytidine
other names
iC (short code)
1-β- D- ribofuranosyl-2-aminopyrimidin-4-one
1 - [(2 R , 3 R , 4 S , 5 R ) -3,4-dihydroxy-5- (hydroxymethyl) oxolan-2-yl] -2-aminopyrimidin-4-one
Molecular formula
C 9 H 13 N 3 O 5
External identifiers / databases
properties
Molar mass
243.22 g mol −1
Physical state
firmly
safety instructions
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions .
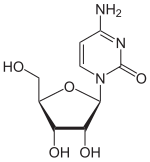
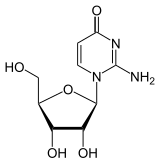
Isocytidine is a synthetic nucleoside . It consists of β- D ribofuranose (sugar) and the nucleobase isocytosine . It is an isomer of cytidine , with the amino group and carbonyl group switching places. The other isomers are pseudocytidine and pseudoisocytidine . Compared to other pyrimidine ribonucleosides, isocytidine can easily be hydrolyzed with dilute acetic acid .
Cytidine, C.
Isocytidine, iC
Individual evidence
↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
↑ N. Kochetkov, Eduard Izrailevich Budovskiĭ, Lord Todd, DM Brown: Organic Chemistry of Nucleic Acids. ISBN 1475705441 p. 432 ( limited preview in Google Book Search).
<img src="https://de.wikipedia.org//de.wikipedia.org/wiki/Special:CentralAutoLogin/start?type=1x1" alt="" title="" width="1" height="1" style="border: none; position: absolute;">