Isocytosine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isocytosine | |||||||||||||||
| other names |
2-amino-pyrimidin-4-one |
|||||||||||||||
| Molecular formula | C 4 H 5 N 3 O | |||||||||||||||
| Brief description |
white prisms |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 111.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
276 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Isocytosine is a heterocyclic organic compound with a pyrimidine backbone. It is an isomer of the nucleobase cytosine , with the amino group and carbonyl group switching places. It is part of the nucleosides isocytidine and pseudoisocytidine .
presentation
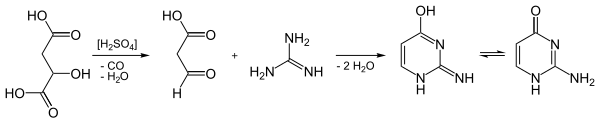
Isocytosine can be obtained by condensing guanidine hydrochloride with 3-oxopropanoic acid ("formyl acetic acid", C 3 H 4 O 3 ). In this case, however, the C 3 component cannot be stored and was therefore replaced by malic acid . This is decarbonylated in concentrated sulfuric acid with elimination of water , so it loses carbon monoxide . The 3-oxopropanoic acid formed in situ condenses with the guanidine in the sulfuric acid solution with double elimination of water.
properties
Isocytosine forms white prisms with a melting point of 276 ° C. It is tautomeric and crystallizes in a 1: 1 ratio of a 1 H and a 3 H variant.
use
Isocytosine is used together with isoguanine to investigate unusual base pairings in DNA .
Individual evidence
- ^ A b c d William T. Caldwell, Harry B. Kime: "A New Synthesis of Isocytosine", J. Am. Chem. Soc. , 1940 , 62 (9), pp. 2365-2365 ( doi : 10.1021 / ja01866a028 ).
- ↑ a b Data sheet Isocytosine, ≥99% from Sigma-Aldrich , accessed on October 31, 2013 ( PDF ).
- ↑ Martin Dračínský, Petr Jansa, Kari Ahonen, Miloš Buděšínský: "Tautomerism and the Protonation / Deprotonation of Isocytosine in Liquid- and Solid-States Studied by NMR Spectroscopy and Theoretical Calculations", European Journal of Organic Chemistry , 2011 , 2011 (8) , Pp. 1544-1551 ( doi : 10.1002 / ejoc.201001534 ).
- ↑ Andrzej Jaworski, Józef S. Kwiatkowski, Bogdan Lesyng: "Why isoguanine and isocytosine are not the components of the genetic code", International Journal of Quantum Chemistry, Supplement: Proceedings of the International Symposium on Quantum Biology and Quantum Pharmacology , 1985 , 28 (Supplement S12), pp. 209-216 ( doi : 10.1002 / qua.560280720 ).
- ↑ Christopher Roberts, Rajanikanth Bandaru, Christopher Switzer: "Theoretical and Experimental Study of Isoguanine and Isocytosine: Base Pairing in an Expanded Genetic System", J. Am. Chem. Soc. , 1997 , 119 (20), pp. 4640-4649 ( doi : 10.1021 / ja970123s ).
- ↑ Xiang-Lei Yang, Hiroshi Sugiyama, Shuji Ikeda, Isao Saito, Andrew H.-J. Wang: "Structural Studies of a Stable Parallel-Stranded DNA Duplex Incorporating Isoguanine: Cytosine and Isocytosine: Guanine Basepairs by Nuclear Magnetic Resonance Spectroscopy," Biophys. J. , 1998 , 75 (3), pp. 1163-1171 ( PMID 9726918 ; PMC 1299791 (free full text); doi : 10.1016 / S0006-3495 (98) 74035-4 ).