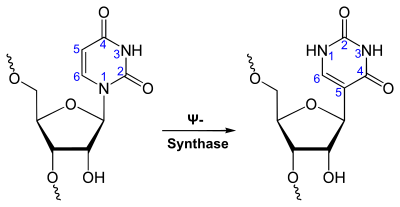Pseudouridine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Pseudouridine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 9 H 12 N 2 O 6 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 244.20 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
220-221 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Pseudouridine (abbreviated from the Greek letter Psi , Ψ ) is a naturally occurring nucleoside . It consists of β- D- ribofuranose (sugar) and the nucleobase uracil . Pseudouridine is an isomer of uridine .
history
Pseudouridine (Ψ) was discovered in the early 1950s as the first naturally modified nucleoside and is one of the most common modified nucleosides. It occurs particularly in tRNA in the TΨC loop.
properties
Pseudouridine forms a base pair with adenosine .
Chemically, it is a C- glycoside , in which the β- D - ribose is linked to the C 5 -atom of the base uracil . In uridine, on the other hand, the ribose is linked to a nitrogen atom in uracil.
Pseudouridine is only formed from uridine after transcription (post-transcriptional) . This isomerization is catalyzed by Ψ-synthases, which is referred to in the literature as pseudouridylation .
See also
literature
- M. Charette, MW Gray: "Pseudouridine in RNA: What, Where, How, and Why", in: IUBMB Life , 2000 , 49 (5), pp. 341-351 ( PMID 10902565 ; doi: 10.1080 / 152165400410182 ).
Individual evidence
- ^ WE Cohn: “Pseudouridine, a Carbon-Carbon Linked Ribonucleoside in Ribonucleic Acids: Isolation, Structure, and Chemical Characteristics”, in: J. Biol. Chem. , 1960 , 235 (5), pp. 1488-1498 ( PMID 13811056 ; PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ WE Cohn and E. Volin: “Nucleoside-5'-Phosphates from Ribonucleic Acid”, in: Nature , 1951 , 167 , pp. 483-484 ( doi: 10.1038 / 167483a0 ).
- ↑ T. Hamma and AR Ferré-D'Amaré: “Pseudouridine Synthases”, in: Chem. Biol. , 2006 , 13 (11), pp. 1125-1135 ( PMID 17113994 ).
- ↑ R. Cortese, HO Kammen, SJ Spengler, BN Ames: “Biosynthesis of Pseudouridine in Transfer Ribonucleic Acid”, in: J. Biol. Chem. , 1974 , 249 (6), pp. 1103-1108 ( PMID 4592259 ; PDF ).
- ↑ AR Ferré-D'Amaré: "RNA-Modifying Enzymes", in: Curr Opin Struct Biol , 2003 , 13 (1), pp. 49-55 ( PMID 12581659 ; doi: 10.1016 / S0959-440X (02) 00002- 7 ).
Web links
- Entry for pseudouridines in the Human Metabolome Database (HMDB) , accessed September 24, 2013.
- Modification Summary of pseudouridine in Modomics database, accessed on January 14, 2014.