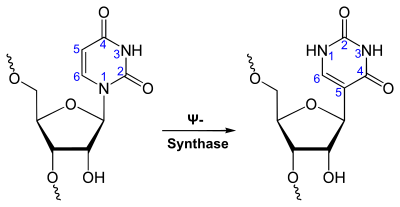Uracil
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Uracil | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 4 N 2 O 2 | ||||||||||||||||||
| Brief description |
white, powdery solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 112.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
334 ° C |
||||||||||||||||||
| solubility |
moderately soluble in cold water, easily soluble in hot water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−429.4 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Uracil (U, Ura) is one of the four most important nucleobases in RNA , along with adenine , cytosine and guanine . Thymine takes its place in the DNA . It is a heterocyclic organic compound with a pyrimidine skeleton and two substituents ( oxygen atoms in positions 2 and 4). The nucleosides of uracil are the uridine in RNA and the very rare deoxyuridine in DNA. In the Watson-Crick base pairing , it forms two hydrogen bonds with adenine.
history
Uracil was isolated in Marburg in 1900 as a cleavage product of the nuclein from yeast by the Italian Alberto Ascoli , advised by his teacher Albrecht Kossel . However, the name for uracil goes to the Leipzig chemist Robert Behrend back of the structure of this compound from the 1885 methyl - derivative was derived - in his attempts descendants of uric acid to synthesize.
presentation
Uracil can be obtained by condensation of urea with 3-oxopropanoic acid ("formyl acetic acid", C 3 H 4 O 3 ). In this case, however, the C 3 component cannot be stored and was therefore replaced by malic acid . This is decarbonylated in concentrated sulfuric acid with elimination of water , so it loses carbon monoxide . The 3-oxopropanoic acid formed in situ condenses with the urea in the sulfuric acid solution with two-fold elimination of water.
properties
Uracil is a white, powdery solid that melts at 341 ° C.
The compound was discovered in the hydrolytic breakdown of nucleic acids. An X-ray crystal structure analysis showed that of the possible tautomeric forms in the solid state (several desmotropic forms exist) the dioxo form is present.
Uracil is a weak acid (pK a = 9.45). The acidity constant is in the range of phenol (pK a = 10.0) and other enols. In the uracil anion, the negative charge is delocalized (mesomeric boundary structures). Therefore, uracil dissolves in aqueous alkalis and aqueous ammonia.
Biological importance
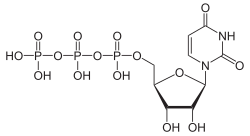
Uracil is mainly bound to ribose phosphate in the body , either as one of the nucleotides uridine monophosphate (UMP), uridine diphosphate (UDP) or uridine triphosphate (UTP) or as a component of ribonucleic acid (RNA).
Part of the RNA
Uracil forms the base pairing to adenine with two hydrogen bonds via the 4-oxo group and the N – H group .
Physiologically, uracil is only incorporated into the single-stranded RNA, but not into the double-stranded deoxyribonucleic acid (DNA). For mating with adenine occurs during the transcription , in the loops ( loops ) of the tRNA and during the translation ( protein synthesis ).
Nucleosides
Uracil forms two different nucleosides with riboses , uridine (U) (linked via the N 1 atom) and pseudouridine (Ψ) (linked via the C 5 atom).
Nucleotides
The phosphorylation of uridine at the C 5 atom of ribose leads to the important nucleotides uridine monophosphate (UMP), uridine diphosphate (UDP) and uridine triphosphate (UTP).
Comparison of uracil and thymine
In RNA, in addition to the purine bases adenine (A) and guanine (G), the two pyrimidine bases cytosine (C) and uracil (U) occur as nucleobases ; in DNA instead of uracil, thymine (T) occurs. A uracil can be produced from a cytosine relatively easily by deamination and hydrolysis , whereby the base sequence is then changed (mutated) and the information genetically encoded in the nucleotide sequence can be changed.
Thymine differs from uracil through an additional methyl group ( 5-methyl-uracil ); therefore it cannot arise quite so easily from cytosine. Uracil occurring in a DNA can be recognized as a mutated base by specific repair enzymes, removed and exchanged for cytosine ( base excision repair ).
Biochemical degradation
Uracil is broken down into carbon dioxide (CO 2 ), two ammonium ions (NH 4 + ) and 3-oxopropanoic acid . The latter reacts further to form malonyl-CoA , which can be used, for example, in fatty acid synthesis .
Related compounds and derivatives

|

|

|

|

|

|
|
|
|
Dihydrouracil |
1-methyluracil |
3-methyluracil |
5-methyluracil (= thymine) |
5-fluorouracil |
5-chloruracil |
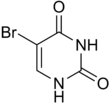
5-bromouracil |
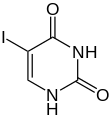
5-ioduracil |
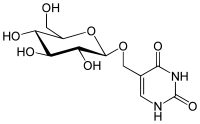
|
|
Glucopyranosyloxymethyluracil |
Individual evidence
- ↑ a b c d data sheet Uracil, 99 +% at AlfaAesar, accessed on December 6, 2019 ( PDF )(JavaScript required) .
- ↑ a b c data sheet uracil (PDF) from Calbiochem, accessed on December 7, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ Alberto Ascoli: About a new cleavage product of yeast nucleins. In: Hoppe-Seyler (ed.): Journal for physiological chemistry . tape 31 , no. 1-2 , October 2, 1900, pp. 161–164 , doi : 10.1515 / bchm2.1901.31.1-2.161 .
- ↑ See also Acknowledgments on p. 164 .
- ↑ Robert Behrend: Attempts to synthesize bodies of the uric acid series. In: Justus Liebig's Annals of Chemistry . tape 229 , no. 1-2 , March 4, 1885, pp. 1-44 , doi : 10.1002 / jlac.18852290102 .
- ↑ RF Stewart, LH Jensen: "Redetermination of the crystal structure of uracil", Acta Cryst , 1947 , 23 , pp. 1102-1105 ( doi: 10.1107 / S0365110X67004360 ).
Web links
- Entry for uracil in the Human Metabolome Database (HMDB) , accessed October 12, 2013.