Backbone (biochemistry)

In the chemistry , in particular the biochemistry , as well as the molecular biology is meant by a backbone (English backbone "backbone"), a main chain or backbone of a continuous series of covalently bonded atoms, which as a chain, the "backbone" of a macromolecule form. The molecular components bound to it, for example side chains, do not belong to the backbone.
Proteins
In the case of proteins , the backbone runs through the peptide bonds that link the amino acids to form the polypeptide chain:
- [H 3 N + - (C α R) - (C = O) - [ NH– (C α R n ) - (C = O) ] n –O -
The torsion angles of the backbone of proteins are denoted by φ, ψ and ω; the former two are shown in the Ramachandran plot . The torsion angles in the side chains are denoted by χ1, χ2, etc. starting from the backbone. When depicting proteins, often only the course of the backbone is shown, with secondary structures often being shown in different colors (see figure).
Nucleic acids

In the case of nucleic acids , the backbone is formed by the alternately linked phosphoric acid and pentose subunits. The nucleobases are each bound to this backbone via the pentose.
With ribonucleic acid (RNA), atoms of the phosphoric acid and ribose subunits form the backbone.
With deoxyribonucleic acid (DNA), atoms of the phosphoric acid and deoxyribose subunits form the backbone. In contrast to other biopolymers, the double-stranded DNA molecule has two opposing backbones.
Polysaccharides
All polysaccharides have a backbone made up of their sugar subunits. With unbranched polysaccharides such. B. Cellulose has no side chains on this backbone.
In the case of branched polysaccharides, a distinction is made between the main chain of the backbone and the side chains attached to it:
Amylopectin contains a main chain of 1,4-α- glycosidically linked D- monosaccharide units of glucose and, based on this, an α-1,6-glycosidically linked side chain on about every 25th glucose residue.
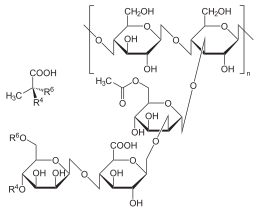
In the case of galactoglucomannan , the backbone consists of 1,4-α- glycosidically linked D- monosaccharide units of glucose and mannose, approximately in a ratio of approximately 2: 1. Similar to the amylose of starch, this main chain adopts a helical screw structure. Α-1,6-glycosidic monomers or short side chains made of sugar residues , which make up about 8% of the molecular mass, are bound to this main chain . This results in a complex, branched-chain polymer, which, however, essentially appears to be unbranched (figures refer to glucomannan from konjac). The "side chains" consist mainly of individual galactose residues .
In the glycogen , glucose building blocks are α-1,4-glycosidically linked, every 8th to 12th of these glucose residues also have a side chain on a 1,6-glycosidic link. Since “side chains” and “main chain” are built up in the same way, the entire molecule can be viewed as a network rather than a backbone.
In the case of xanthan , the backbone consists of β- (1 → 4) -linked D -glucose units. Every second glucose unit is α - (1 → 3) -glycosidic a β - D -mannopyranosyl- (1 → 4) - β - D -glucuronopyranosyl- (1 → 2) -6- O -acetyl- α - D -mannopyranosyl -Side chain knotted.
Individual evidence
- ^ R. Merkl & S. Waack: Bioinformatik Interaktiv. 2nd edition, Wiley-VCH, Weinheim 2009, p. 13, ISBN 978-3-527-32594-8 .
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer, Gregory J. Gatto, Jr.: Stryer Biochemistry . Springer-Verlag, 2014, ISBN 978-3-8274-2988-9 , pp. 34 .
- ↑ Hans-Dieter Jakubke and Hans Jeschkeit: Amino acids, peptides, proteins , Verlag Chemie, Weinheim, 1982 , pp. 96–97, ISBN 3-527-25892-2 .
- ↑ Kaname Katsuraya, Kohsaku Okuyamab, Kenichi Hatanakab, Ryuichi Oshimab, Takaya Satoc, and Kei Matsuzakic (2003). Constitution of konjac glucomannan: chemical analysis and 13C NMR spectroscopy. In: Carbohydrate Polymers . 53 (2), pp. 183-189; doi: 10.1016 / S0144-8617 (03) 00039-0 .
- ↑ E. Gruber: Fundamentals of pulp technology , Fig. 8 and 9.
- ^ Entry on (1-6) -alpha-glucomannan at ChemicalBook , accessed December 30, 2011.
- ↑ Tea Hannuksela, Catherine Hervé du Penhoat: NMR structural determination of dissolved O-acetylated galactoglucomannan isolated from spruce thermomechanical pulp . In: Carbohydrate Research . tape 339 , no. 2 , January 2004, p. 301-312 , doi : 10.1016 / j.carres.2003.10.025 .