Pentoses
Pentoses (from the Greek pente five) are monosaccharides with a carbon structure containing five carbon atoms . In non-reduced form they have the empirical formula C 5 H 10 O 5 and differ in the type of carbonyl function . If it is a keto group , then one speaks of ketopentoses , in the case of an aldehyde group it is called aldopentoses .
The aldopentoses
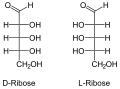
The aldopentoses have three chiral centers , so there are eight stereoisomers . Of these, only D - ribose , D - xylose , D - arabinose and L - arabinose occur in nature . D - and L - lyxose does not occur naturally in free form. The D / L configuration in aldopentoses always relates to the position of the OH group on the 4th carbon atom and has no relation to the direction of rotation of the optical activity.
- Aldopentoses in Fischer projection
Due to the intramolecular hemiacetal formation, these open-chain molecules are in equilibrium with their pyranose or furanose forms. This creates an oxygen bridge between the 1st and 5th carbon atoms (pyranose) or the 1st and 4th carbon atoms (furanose), and the oxygen atom of the aldehyde group becomes the hydroxyl group.
The ketopentoses
The ketopentoses (synonym pentuloses ) have two chiral centers , so there are four stereoisomers . The D / L configuration relates - as above - to the position of the OH group in the fourth carbon atom, and has no relation to the sign [(+) or (-)] of the rotation value α. Only the two D-isomers occur in nature.
- Ketopentoses in Fischer projection
Pentoses and their biological significance
The D - ribose and its reduced form, the D - deoxyribose are essential components of the RNA or DNA .
The D- ribulose serves as a CO 2 acceptor in photosynthesis.
The metabolism of pentoses and their formation through the decarboxylation of hexoses is summarized in the pentose phosphate cycle . The key reaction is the direct oxidation of glucose-6-phosphate by the enzyme glucose-6-phosphate dehydrogenase , whose gene is located on the X chromosome in humans. Defects can cause a number of clinical pictures (e.g. in blood formation) and overreactions to certain drugs.
See also
Individual evidence
- ↑ Paula Y. Bruice: Organic Chemistry. 5th updated edition. Pearson Studium, Munich et al. 2007, ISBN 978-3-8273-7190-4 , pp. 1118–1119.