Xylose
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Xylose | ||||||||||||||||||
| other names |
Wood sugar |
||||||||||||||||||
| Molecular formula | C 5 H 10 O 5 | ||||||||||||||||||
| Brief description |
colorless, sweet-tasting needles or prisms |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 150.13 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
154–158 ° C (both enantiomers) |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Xylose ( wood sugar , from ancient Greek ξύλον xylon , German , wood ' ) is a Aldo pentose , a type of sugar with five carbon - atoms and an aldehyde group as a functional group which, inter alia, in the hydrolysis of wood rubber with dilute acids is produced. The sugar substitute xylitol is made from it. In relation to sucrose , a 10% D -Xylose solution has a sweetness of 67%. Xylose is not broken down in the human organism and is excreted again in unchanged form. In medicine, xylose is therefore used to investigate gastric emptying and resorption processes in the intestine.
Stereochemistry
The term xylose stands for two enantiomers : D- xylose and L- xylose, which relate to each other like image and mirror image. The position of the hydroxyl group at C 4 (the second lowest carbon atom) determines whether it is the D or the L enantiomer. In the Fischer projection , this points to the right in D -Xylose ( Latin : dexter, hence D ), in L -Xylose to the left (Latin: laevus, L ). Like other long-chain sugars , xylose can react intramolecularly to form a cyclic hemiacetal with five or six atoms in the ring. Due to the newly created chiral center, there are four isomers each for the ring forms of D - and L -xylose.
| D -Xylose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
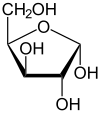 α- D -xylofuranose |
 β- D -xylofuranose |
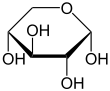 α- D -Xylopyranosis |
 β- D -Xylopyranosis |
|
The suffixes -pyranose and -furanose describe the similarity of the ring with the heterocycles pyran and furan .
At 20 ° C, D -xylose dissolved in water is, like D - glucose , 35% in the α-pyranose form and 65% in the β-pyranose form.
The CAS number for the open-chain, racemic xylose is 25990-60-7 . Each ring shape and each stereoisomer has its own CAS number:
| open- chain |
Furanosis | Pyranosis | |||
|---|---|---|---|---|---|
| α | β | α | β | ||
| D -Xylose | 58-86-6 | 14795-83-6 | 37110-85-3 | 6763-34-4 | 2460-44-8 |
| L -Xylose | 609-06-3 | 41546-30-9 | 41546-29-6 | 7296-58-4 | 7322-30-7 |
Medical use
The D -Xylose Absorption Test can be used to test for malabsorption .
Web links
Individual evidence
- ↑ a b c Entry on xylose. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ L-Xylose data sheet from Sigma-Aldrich , accessed on May 12, 2017 ( PDF ).
- ↑ Data sheet D-Xylose from Sigma-Aldrich , accessed on May 12, 2017 ( PDF ).
- ↑ a b data sheet DL-Xylose at Sigma-Aldrich , accessed on October 16, 2016 ( PDF ).
- ↑ Hans-Dieter Belitz, Werner Grosch and Peter Schieberle : Textbook of food chemistry . Springer, Berlin; 6th, completely revised edition 2008; ISBN 978-3-540-73201-3 ; P. 263.
- ↑ Hans-Dieter Belitz , Werner Grosch and Peter Schieberle: Textbook of food chemistry . Springer, Berlin; 6th, completely revised edition 2008; ISBN 978-3-540-73201-3 ; P. 269.