Xylitol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
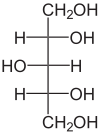
| Xylitol in the Fischer projection | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Xylitol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 12 O 5 | |||||||||||||||||||||
| Brief description |
colorless, sweet-tasting crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 152.15 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.52 g / cm −3 |
|||||||||||||||||||||
| Melting point |
94 ° C |
|||||||||||||||||||||
| boiling point |
216 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Xylitol (also xylitol ) is a common name for a stereoisomer of the sugar alcohol pentane pentol . As a food additive , it bears the designation ( E 967) and serves as a sugar substitute . The discovery goes back to the later Nobel Prize winner Emil Fischer .
The peculiarity of xylitol is its proven in several clinical trials cariostatic and anti-cariogenic effect. On the other hand, xylitol has a toxic effect on some mammals, especially dogs, which leads to its importance in veterinary medicine .
discovery
Xylitol was first isolated from beech wood shavings by Emil Fischer and his doctoral student Rudolf Stahel around 1890 , cf. ancient Greek ξύλον xýlon , German 'wood' . They published their discovery, which they called xylitol , in 1891. Around the same time as Fischer and Stahel, the French chemist MG Bertrand isolated a type of xylitol syrup from wheat and oat stalks.
Occurrence and manufacture
In addition to sorbitol, xylitol is a natural sugar alcohol component of many types of vegetables such as a. Cauliflower and fruits such as plums , strawberries or raspberries , the proportion being less than 1% of the dry matter . Also in the bark of certain types of wood such as B. Birch and beech contain xylitol.
Contrary to what the alternative name birch sugar might suggest, xylitol is not obtained directly from birch wood. The industrial production of xylitol is based on xylans ( wooden rubber ) from birch wood and other hardwoods or agricultural residues such as corn cobs (corn cobs), straw, grain bran or sugar cane bagasse . From these, xylose is released at temperatures of up to 200 ° C and using sulfuric acid or sodium hydroxide solution. The xylose is then converted to xylitol with a catalyst under high pressure. Biotechnological processes such as the use of yeasts such as Candida tropicalis are possible, but are not yet used on an industrial scale. Industrial production is complex, which is why xylitol is a relatively expensive sugar substitute.
As an intermediate product in the human body during the breakdown of carbohydrates, 5 to 15 grams of xylitol are produced daily in the liver .
properties
Chemically, xylitol is a pentitol and belongs to the sugar alcohols. The molecule is pseudo-asymmetrical and has an achiral meso form . The other achiral form of pentitol is ribitol , the chiral form arabitol .
Xylitol has a similar taste and almost the same sweetness (98%) as sucrose . If xylitol dissolves in the saliva in the mouth, it removes heat from the environment and creates a cooling effect ( endothermic heat of solution ) of −153.2 J / g on the tongue , which is described in a similar way to the effect of menthol .
The physiological calorific value of 10 kJ / g (2.4 kcal / g) is 40% lower than that of household sugar (sucrose). The metabolic pathway in the human body is almost independent of insulin. If insulin values of approx. 110 pmol / L are reached with sucrose, it is only 50 pmol / L with xylitol. It therefore has less of an effect on blood sugar and insulin levels than sucrose. Xylitol is therefore used as a substitute for sucrose by people with diabetes mellitus .
Xylitol is heat-stable and only caramelises if it is heated to over 200 ° C for several minutes. At temperatures around 100 ° C there is no caramelization. In the pure state it is in the form of hygroscopic crystals.
Xylitol can be used instead of sugar in both baking and cooking, it has no off-flavors. The consistency is very similar, but xylitol is more soluble when hot than cold. The only restriction: yeast doughs do not rise with xylitol. You should also refrain from mixing the sugar substitute with other sweeteners such as aspartame , saccharin or sorbitol - it may then no longer be well tolerated.
Xylitol is a molecule that can bind a lot of water. It is only passively absorbed in the small intestine, i.e. slowly and incompletely. If taken regularly, the rate of absorption in the small intestine can be increased by enzyme induction . If more than 0.5 g xylitol per kg body weight is taken, a laxative effect can occur, which can disappear after the organism has adapted (after 3–4 weeks of getting used to it). In the course of studies, the intake of 200 g xylitol daily was tolerated without any problems. This adaptation does not exist with sorbitol , so sorbitol always has a laxative effect.
In the large intestine, the remaining xylitol (about 2/3 of the ingested amount) is broken down by bacteria and broken down into small fatty acid components and absorbed. These are metabolized into carbon dioxide (CO 2 ) and water.
Medical importance
Anti-cariogenic effect
Studies
In the early 1970s, the caries-reducing properties of sugar alcohol were discovered. At the University of Turku (Finland), two clinical studies (known as the Turku Sugar Studies) were carried out between 1972 and 1975 that demonstrated a highly significant reduction in tooth decay.
In the first two-year nutritional study, sugar (sucrose) was replaced by fructose or xylitol in all foods . A total of 115 people in 3 groups took part. The consumption of the sweeteners was 50 to 67 g per day. According to the study, a caries reduction of 30% with fructose and over 85% with the use of xylitol could be determined. The so-called DMFS index was used for comparison. The increase in the index was 7.2 in the sucrose group, 3.8 in the fructose group and 0.0 in the xylitol group.
The second study was started after a marked reduction in DMFS values was observed in subjects during the first study, i.e. This means that they exhibited a so-called caries reversal, whereby certain caries lesions had undergone a re-hardening process. The xylitol strengthens the physiologically occurring re-hardening process by preventing the production of acids from other food sugars. Around 100 people were divided into sucrose and xylitol groups. The sweeteners were given in chewing gum for a year, about 7 grams per person per day. Compared to the sucrose group, a reduction in the rate of caries growth of more than 82% was found in the xylitol test persons. The chewing effect could be ruled out because both groups consumed the same amount of chewing gum. One conclusion of the study is that even small amounts of xylitol are sufficient and a complete change in the sweetener is not necessary.
These effects are explained by the fact that the cariogenic bacteria of the species Streptococcus mutans cannot metabolize xylitol and thus die. Furthermore, they are also prevented from adhering to the tooth surface as plaque bacteria . The optimal amount of xylitol was determined to be between 5 and 10 grams per day in several servings. This can be absorbed using chewing gum or lozenges.
In addition, xylitol stimulates the production of saliva and promotes the formation of complexes with calcium and saliva proteins in the oral cavity, which leads to a remineralization of tooth structure. Xylitol increases the pH value in the oral cavity, which is a basic requirement for calcium storage in tooth enamel.
Another Turku study from 2000 examined the interactions between mothers who regularly chewed chewing gum containing xylitol and their children (up to 2 years old). One result of the study was that the regular consumption of xylitol chewing gum by the mothers significantly inhibited the infestation with Streptococcus mutans in the children.
A double-blind study from 2013 with 538 people who received 5 grams of xylitol per day, however, found no preventive effect against tooth decay in patients with sufficient fluoride intake.
A meta-study by the Cochrane Collaboration from 2015 sees evidence of a 13% reduction in tooth decay in children by using fluoride toothpaste with xylitol (compared to toothpaste containing only fluoride). Conclusions about a preventive effect of other xylitol-containing products are not possible due to the study situation. Overall, the quality of the documents is rated as low to very low.
Mechanism and Resistance
Xylitol works in Streptococcus mutans through two mechanisms:
- Inhibition of the first half of glycolysis . This effect is synergistically enhanced by fluoride . Glycolysis is inhibited by competitive inhibition of phosphofructokinase by xylitol-5-phosphate or xylulose-5-phosphate, which is produced by phosphorylation when xylitol is imported.
- Xylitol enters a “ senseless cycle of import / phosphorylation, dephosphorylation and subsequent export ”, whereby PEP and nicotinamide adenine dinucleotide (NAD + ) are consumed by the phosphotransferase system during import and ATP may be consumed during export.
It is assumed that not all strains of S. mutans are sensitive to xylitol and that resistant strains can therefore reproduce preferentially when xylitol is used. However, it is also assumed that these are less cariogenic. The resistance effect is not as pronounced as with antibiotics , since xylitol has another effect, namely the reduction of the biofilm , the mucous layer on the teeth and in the oral cavity, which is caused by a lack of carbohydrates and chewing gum. As a result, even patients with mainly resistant strains have a reduced colonization.
Prophylaxis of acute otitis media
In some studies, the administration of high doses of xylitol was able to achieve a preventive effect against acute otitis media acute . Xylitol inhibits the growth of pneumococci and the binding of pneumococci and Haemophilus influenzae to cells in the nasopharynx . The xylitol dose was in the range of 10 g / day.
Veterinary importance
In some animal species (dogs, cattle, goats, rabbits) xylitol has a strong insulin-releasing effect, which can lead to a life-threatening drop in blood sugar levels ( hypoglycaemia ). Severe liver damage up to liver failure and clotting disorders have also been observed in dogs . Even a dose of 0.1 g per kg of body mass is toxic to the animal, a lethal dose is reached from approx. 3-4 g of xylitol per kg of body weight. Consuming a bag of xylitol-containing candy can be fatal, even for a large dog, if the animal does not receive intensive care as soon as possible.
Products containing xylitol are generally safe for cats. In a study on cats, the positive effects of xylitol on their oral hygiene were proven. If cats are given xylitol-enriched water, this will significantly reduce tartar and plaque.
literature
- EM Söderling: Xylitol, mutans streptococci, and dental plaque. In: Advances in dental research. Volume 21, number 1, 2009, pp. 74-78, doi: 10.1177 / 0895937409335642 . PMID 19717413 . (Review).
- C. Hayes: The effect of non-cariogenic sweeteners on the prevention of dental caries: a review of the evidence. In: J Dent Educ. Volume 65, 2001, pp. 1106-1109. PMID 11699985 . jdentaled.org (PDF)
- Laura E. Berk: Developmental Psychology. Pearson Studium, 2005, pp. 288-289.
- Kauko K. Mäkinen: The use of xylitol in caries prophylaxis . pdv Praxis-Dienst und Verlag, Heidelberg 2003, ISBN 3-935802-09-9 . xylismile.de (PDF)
- Wolfgang Strübig: Xylitol and chewing gum - an ideal combination to prevent caries? In: Dental Hygiene Journal. No. 4, 2005, pp. 33-37.
- Z. Gintner, J. Szöke, A. Patthy, E. Söderling, J. Banoczy: Effect of xylitol lozenges on dental plaque and Streptococcus mutans. In: Oral prophylaxis & pediatric dentistry. Volume 26, 2004, pp. 93-95. zahnheilkunde.de (PDF).
Web links
- E 967 (xylitol) . Zusatzstoffe-online.de
Individual evidence
- ↑ Entry on E 967: Xylitol in the European database for food additives, accessed on June 27, 2020.
- ↑ a b Birch sugar - nothing else than the additive xylitol. Lebensmittelklarheit.de, June 27, 2016, accessed on July 13, 2016.
- ↑ Entry on XYLITOL in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d Entry on xylitol. In: Römpp Online . Georg Thieme Verlag, accessed on June 26, 2014.
- ↑ a b Xylitol data sheet (PDF) from Fisher Scientific , accessed on February 13, 2014.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-522.
- ↑ a b Data sheet Xylitol, 99% from AlfaAesar, accessed on December 21, 2019 ( PDF )(JavaScript required) .
- ↑ Entry on xylitol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ E. Fischer, R. Stahel: On the knowledge of xylose. In: Reports of the German Chemical Society. Volume 24, Number 1, 1891, pp. 528-539; doi: 10.1002 / cber.189102401100 .
- ^ MG Bertrand: Research on the quelques dérivés du xylose. In: Bull Soc Chim Paris. Volume 5, 1891, pp. 554-557.
- ↑ KK Mäkinen: The use of xylitol in caries prophylaxis. ( Memento from November 19, 2011 in the Internet Archive ) (PDF; 1.8 MB). 2003, ISBN 3-935802-09-9 , p. 9.
- ↑ KK Mäkinen: The rocky road of xylitol to its clinical application. In: Journal of Dental Research . Volume 79, Number 6, June 2000, pp. 1352-1355, PMID 10890712 .
- ↑ Information from Karl Herrmann: Ingredients of fruit and vegetables: 50 tables and overviews. Ulmer, Stuttgart (Hohenheim) 2001, ISBN 3-8001-3139-0 .
- ^ Hans-Dieter Belitz, Werner Grosch, Peter Schieberle: Textbook of food chemistry. 6th, completely revised edition. Springer, Berlin 2008, ISBN 978-3-540-73201-3 , p. 263.
- ↑ Metabolic response to lactitol and xylitol in healthy men1 (PDF; 1.07 MB).
- ↑ What should be considered when using xylitol when cooking and baking
- ↑ The DMFS index describes the life history of total caries processes in a person. Here: D (decayed) stands for the increase in the number of carious tooth surfaces; M (missing) due to extraction of missing teeth; F (filled) filled tooth surfaces; S (surface) Number of tooth surfaces. The index has been criticized because it only measures the increase in caries on healthy and filling-free tooth surfaces.
- ↑ The use of xylitol in caries prophylaxis ( Memento from July 22, 2014 in the Internet Archive ).
- ↑ Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age . April 13, 2019 (English), PMID 11145360
- ↑ John P. Brown, Bennett T. Amaechi, et al. a .: Visual scoring of non cavitated caries lesions and clinical trial efficiency, testing xylitol in caries-active adults. In: Community Dentistry and Oral Epidemiology. 2013, pp. N / a – n / a, doi: 10.1111 / cdoe.12082 .
- ↑ P. Riley, D. Moore, F. Ahmed, MO Sharif, HV Worthington: Xylitol-containing products for preventing dental caries in children and adults . In: The Cochrane Library . No. 3 , March 26, 2015, doi : 10.1002 / 14651858.CD010743.pub2 (English, Art. No .: CD010743).
- ↑ Philip Marsh, Michael V. Martin: Orale Mikrobiologie. ISBN 978-3-13-129731-0 , p. 117, limited preview in Google Book Search.
- ^ SZ Hausman, J. Thompson, J. London: Futile xylitol cycle in Lactobacillus casei. In: Journal of bacteriology. Volume 160, Number 1, October 1984, pp. 211-215. PMID 6090413 . PMC 214702 (free full text).
- ↑ EM Söderling: Xylitol, mutans streptococci, and dental plaque. In: Advances in dental research. Volume 21, number 1, 2009, pp. 74-78, doi: 10.1177 / 0895937409335642 . PMID 19717413 .
- ↑ JL Danhauer, CE Johnson a. a .: Xylitol as a prophylaxis for acute otitis media: systematic review. In: International journal of audiology. Volume 49, number 10, October 2010, pp. 754-761, doi: 10.3109 / 14992027.2010.493897 . PMID 20874048 . (Review).
- ↑ M. Uhari, T. Kontiokari, M. Niemelä: A novel use of xylitol sugar in preventing acute otitis media. In: Pediatrics. Volume 102, Number 4 Pt 1, October 1998, pp. 879-884. PMID 9755259 .
- ^ JL Danhauer, A. Kelly, CE Johnson: Is mother-child transmission a possible vehicle for xylitol prophylaxis in acute otitis media? In: International journal of audiology. Volume 50, number 10, October 2011, pp. 661-672, doi: 10.3109 / 14992027.2011.590824 . PMID 21812632 . (Review).
- ↑ M. Uhari, T. Tapiainen, T. Kontiokari: Xylitol in preventing acute otitis media. In: Vaccine. Volume 19 Suppl 1, December 2000, pp. S144-S147. PMID 11163479 .
- ↑ EK Dunayer, SM Gwaltney-Brant: Acute hepatic failure and coagulopathy associated with xylitol ingestion in eight dogs. In: Journal of the American Veterinary Medical Association. Volume 229, Number 7, October 2006, pp. 1113-1117, doi: 10.2460 / javma.229.7.1113 . PMID 17014359 .
- ↑ Xylitol in dogs and small animals.
- ^ ME Peterson: Xylitol. In: Topics in companion animal medicine. 28 (1), 2013, pp. 18-20. "Xylitol containing products are safe for cats, and it has been proposed to add it to daily water to prevent feline dental disease [...]"
- ↑ DE Clarke: Drinking Water Additive Decreases Plaque and Calculus. In: J'Vet Dent. 23 (2), 2006, pp. 79-82.

