Fluoride
Fluorides are the salts of hydrofluoric acid (HF), also known as hydrofluoric acid. They contain fluoride ions (F - ) in their ion lattice as negative lattice building blocks ( anions ). In addition, covalent , non-ionic compounds of non-metals and organic fluorine compounds such as fluorocarbons or carboxylic acid derivatives are outdated and referred to as fluorides.
Natural occurrence
Fluorides occur naturally and in the human body in the form of many minerals . The most important representative is fluorite (CaF 2 ), furthermore yttrofluorite , an addition mixed crystal with YF 3 , and cerium fluorite (also yttrocerite ), which in addition to yttrium also contains cerium , erbium and water of hydration. Further fluorides are frankdicksonite (BaF 2 ), gagarinite (NaCaYF 6 ), tysonite (also fluocerite , (Ce, La, Se) F 3 ) and neighborite (NaMgF 3 ). Complex fluorides contain another element within an anion complex, such as boron , aluminum or silicon , and then form e.g. B. hexafluorosilicates or tetrafluoroborates . Representatives here are ferruccite (NaBF 4 ), avogadrite ((K, Cs) BF 4 ), malladrite (Na 2 SiF 6 ), hieratite (K 2 SiF 6 ), cryolite ionite (Na 3 Al 2 Li 3 F 12 ), cryolite (Na 3 AlF 6 ), elpasolite (K 2 Na [AlF 6 ]), jarlite (NaSr 2 [AlF 6 ] 2 ), usovite (Ba 2 Mg [AlF 6 ] 2 ) and weberite (Na 2 MgAlF 7 ).
Inorganic fluorides
Important salty fluorides
Some important fluorides are:
- Aluminum fluoride (AlF 3 )
- Ammonium fluoride (NH 4 F)
- Calcium fluoride (CaF 2 , fluorite or fluorspar)
- Sodium fluoride (NaF)
- Tin (II) fluoride (SnF 2 )
Hydrogendifluoride
In addition to the simple fluorides, there are also hydrogen difluorides with the composition Me + [HF 2 ] - , such as sodium hydrogen difluoride (NaHF 2 ) and potassium hydrogen difluoride (KHF 2 ). These contain the linear [FHF] - anion. It can be prepared from aqueous solutions of the fluoride in the presence of an excess of hydrogen fluoride (HF). When heated, the hydrogen difluorides split off the hydrogen fluoride again.
Presentation:
Decomposition by heating:
Molecularly structured inorganic fluorine compounds such as the hexafluorides platinum (VI) fluoride , uranium (VI) fluoride or plutonium (VI) fluoride are often referred to as fluorides.
Organic fluorides
In most organic fluorides, the fluorine atom is covalently bonded, examples:
- Sarin (C 4 H 10 FO 2 P),
- Tetrafluoromethane (CF 4 )
- Trifluoromethane (CHF 3 )
- Chlorodifluoromethane (CHClF 2 )
However, there are also organic fluorine compounds in which the fluorine atom is contained in the form of a salt as a fluoride anion. Examples:
proof
Physical analysis methods
Today, fluorides in minerals and solids are professionally determined with X-ray fluorescence analysis , X-ray diffraction or mass spectrometry , in liquids with fluoride electrodes , IR or NMR methods.
Wet chemical
With simple laboratory methods, fluoride can be detected by the lead crucible test or the etching sample. If sulfuric acid is added to a sample containing fluoride, hydrogen fluoride is produced , which etches the glass container.
If the sample is placed in a lead crucible with powdered silica or sodium silicate and then covered with sulfuric acid , silicon tetrafluoride gas is formed:
The crucible is closed again and the cover moistened with water. The silicon tetrafluoride reacts with the water again to form silicate , which is deposited in the form of a crater in the water droplets.
Applications
Fluorides are mainly used as a flux in metallurgy , for the synthesis of organic fluorochemicals and for gas-tight sealing of fuel tanks; the plastic tanks are made of z. B. PA ( polyamide ) vaporized with the dissolved fluoride, this diffuses about 3–4 micrometers into the surface.
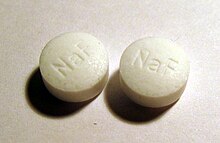
As fluoridation refers to the addition of fluorides in particular to table salt , drinking water , milk , tablets and toothpastes for prevention of dental caries .
According to an opinion of the European Food Safety Authority (EFSA) published in 2013, fluoride is not an essential nutrient because it does not serve growth processes or tooth development and signs of a fluoride deficiency could not be identified. Fluoride can both topically and systemically administered the caries prevention support. Although fluoride has been used to prevent tooth decay for decades, its actual mechanism of action is unknown. It is assumed that the presence of fluorides in the oral cavity leads to an “accelerated” remineralization with calcium and phosphate ions from the saliva. A review article from 2010 indicates that fluorides are only effective from a concentration of 1000 ppm. As a result, children's toothpastes with 500 ppm have no significant effect compared to toothpaste without an active ingredient ( placebo ).
toxicology
The toxicology of fluorides depends on numerous factors, such as the type of fluoride, its solubility behavior, the type of exposure, the rate of absorption in the stomach, the acid-base balance and the pH value of the fluoride ingested. In the case of oral intake (small amounts of fluoride are found in drinking water / mineral water, for example), soluble fluoride is absorbed quickly and almost completely through the gastric mucosa , as the salts form hydrogen fluoride through the hydrochloric acid in the stomach , which is quickly absorbed as an uncharged molecule.
The certainly toxic dose (CTD) is 32 to 64 mg fluoride per kilogram of body weight, in children it is 16 mg / kg. For young children, the probably toxic dose (PTD) is 5 mg fluoride per kilogram body weight.
- For comparison: A tube of toothpaste (100 g or 75 ml) with a fluoride content of 1000 ppm ( parts per million ) contains 100 mg of fluoride. A child weighing 15 kg would have exceeded the likely toxic dose if the entire tube of toothpaste had been consumed.
Chronic, however, a significantly lower dose can be toxic.
According to the latest findings, intake by pregnant women can also reduce the IQ of later children (aged 3–4 years) in normal amounts (4.5 IQ points less for boys per 1 mg / L fluoride in the mother's urine during pregnancy; 3.66 IQ Points per 1 mg of average daily maternal intake during pregnancy).
Water-insoluble or sparingly soluble fluorides such as calcium fluoride and aluminum fluoride have a significantly lower toxicity. However, there is always the risk of the formation of highly toxic hydrogen fluoride on contact with strong acids.
The toxic effect ( fluorosis ) is based partly on the precipitation of the calcium required by the metabolism as calcium fluoride , and partly on the effect of protoplasm and cell toxin , which inhibits certain enzyme systems and protein synthesis. It manifests itself in damage to the skeleton, teeth, lung function, skin and metabolic disorders.
Symptoms of acute fluoride poisoning are severe pain in the stomach and intestines and behind the breastbone, cramps, loss of consciousness and severe metabolic disorders. In higher concentrations, hydrogen fluoride causes severe burns and even destroys cells.
Calcium gluconate serves as an antidote for fluoride poisoning . Other calcium-containing agents are also effective as first aid measures; For example, it can help to drink milk to inhibit the absorption of the fluoride ions.
See also
Individual evidence
- ↑ a b Wissenschaft-Online-Lexika: Entry on Fluoride , in: Lexikon der Geologie, accessed on July 10, 2008.
- ↑ Biltz-Klemm-Fischer, 1966.
- ^ A b Matthias Epple, Joachim Enax: Modern dental care from a chemical point of view . In: Chemistry in Our Time . tape 52 , no. 4 , 2018, p. 218–228 , doi : 10.1002 / ciuz.201800796 .
- ^ Tanya Walsh, Helen V. Worthington, Anne-Marie Glenny, Priscilla Appelbe, Valeria CC Marinho: Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents . In: Cochrane Database of Systematic Reviews . January 20, 2010, ISSN 1465-1858 , doi : 10.1002 / 14651858.cd007868.pub2 .
- ↑ E. Hellwig, J. Klimek , T. Attin: Introduction to tooth preservation - examination knowledge of cariology, endodontology and periodontology. 6th edition. Deutscher Zahnärzteverlag, 2013, pp. 145ff
- ^ GM Whitford: The Metabolism and Toxicity of Fluoride . Monographs in Oral Science (Editor Howard M. Myers) Vol. 13, Karger, Basel 1989
- ↑ Oskar Eichler et al .: Pharmacology of Fluorides , Handbook of Experimental Pharmacology, Vol. XX.1 and XX.2, Springer Verlag, Berlin-Heidelberg-New York, 1966 and 1970
- ↑ Rivka Green, Bruce Lanphear, Richard Hornung, David Flora, E. Angeles Martinez-Mier: Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada . In: JAMA Pediatrics . August 19, 2019, ISSN 2168-6203 , doi : 10.1001 / jamapediatrics.2019.1729 ( jamanetwork.com [accessed August 21, 2019]).
- ^ Philippe Grandjean, Philip J. Landrigan: Neurobehavioural effects of developmental toxicity. The Lancet , Vol. 13, 3, pp. 330-338, March 1, 2014. doi : 10.1016 / S1474-4422 (13) 70278-3
- ^ Entry on antidote. In: Römpp Online . Georg Thieme Verlag, accessed on November 15, 2013.
- ↑ Entry on Gluconate. In: Römpp Online . Georg Thieme Verlag, accessed on November 15, 2013.
- ↑ Jürgen Stein (Hrsg.): Praxishandbuch clinical nutrition and infusion therapy. Springer 2003, ISBN 978-3-642-55896-2 , p. 120, limited preview in the Google book search