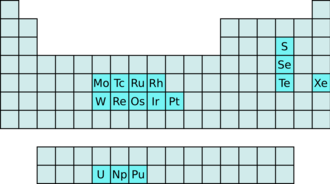
Hexafluorides
The hexafluorides are a group of chemical compounds with the empirical formula XF 6 . Stable hexafluorides are known of 16 elements. 9 of these elements belong to the transition metals , 3 to the actinides , and 4 are non-metals .
properties
Physical Properties
Most hexafluorides are covalent compounds with low melting and boiling points. 4 Hexafluorides (S, Se, Te, W) are gaseous at room temperature (25 ° C) and normal pressure (1013 mbar), 2 are liquid (Re, Mo), the others are volatile solids. 4 Hexafluorides (S, Se, Te, U) change directly into the gaseous state by sublimation when heated . The hexafluorides of the main group (S, Se, Te, Xe) and the 6th subgroup (Mo, W) are colorless, the colors of the other hexafluorides range from yellow to orange, red, brown and black.
| connection |
M.p. (° C) |
Bp (° C) |
Sublp. (° C) |
Physical state |
Molar mass (g mol −1 ) |
Density (g cm −3 ) |
Bond length ( pm ) |
colour |
|---|---|---|---|---|---|---|---|---|
| Sulfur hexafluoride | −63.8 | gaseous | 146.05 | 6.63 kg m −3 | 156.4 | colorless | ||
| Selenium hexafluoride | −46.6 | gaseous | 192.95 | 8.69 kg m −3 | 167-170 | colorless | ||
| Tellurium hexafluoride | −38.9 | gaseous | 241.59 | 3.16 (−40 ° C) | 184 | colorless | ||
| Xenon hexafluoride | 49.48 | 75.6 | firmly | 245.28 | 3.56 | colorless | ||
| Molybdenum hexafluoride | 17.5 | 34.0 | liquid | 209.94 | 3.50 (−140 ° C) | 181.7 | colorless | |
| Technetium hexafluoride | 37.4 | 55.3 | firmly | 212 ( 98 Tc) | 3.58 (−140 ° C) | 181.2 | yellow | |
| Ruthenium hexafluoride | 54 | firmly | 215.07 | 3.68 (−140 ° C) | 181.8 | dark brown | ||
| Rhodium hexafluoride | ≈ 70 | firmly | 216.91 | 3.71 (−140 ° C) | 182.4 | black | ||
| Tungsten hexafluoride | 2.3 | 17.1 | gaseous | 297.84 | 4.86 (−140 ° C) | 182.6 | colorless | |
| Rhenium hexafluoride | 18.5 | 33.7 | liquid | 300.20 | 4.94 (−140 ° C) | 182.6 | yellow | |
| Osmium hexafluoride | 33.4 | 47.5 | firmly | 304.22 | 5.09 (−140 ° C) | 182.9 | yellow | |
| Iridium hexafluoride | 44 | 53.6 | firmly | 306.21 | 5.11 (−140 ° C) | 183.4 | yellow | |
| Platinum hexafluoride | 61.3 | 69.1 | firmly | 309.07 | 5.21 (−140 ° C) | 184.8 | deep red | |
| Uranium hexafluoride | 56.5 | firmly | 351.99 ( 238 U) | 5.09 | 199.6 | colorless | ||
| Neptunium hexafluoride | 54.4 | 55.18 | firmly | 351.04 ( 237 np ) | 198.1 | orange | ||
| Plutonium hexafluoride | 52 | 62 | firmly | 358.06 ( 244 Pu) | 5.08 | 197.1 | brown |
Molecular structure
The molecular geometry is usually octahedral , with the exception of xenon hexafluoride . The connection is square-bipyramidal (distorted octahedral). According to the VSEPR theory, the structure forms a pentagonal-pyramidal molecule due to the remaining free electron pair. Based on quantum chemical calculations, ReF 6 and RuF 6 should have tetragonally distorted structures (in which two of the bonds on one axis are longer or shorter than those of the other four), but this has not yet been observed.
Chemical properties
The hexafluorides offer a wide range of chemical reactivity. Sulfur hexafluoride is almost inert and non-toxic. Due to its stability, dielectric properties and high density, it has numerous applications. Selenium hexafluoride is almost as unreactive as SF 6 , whereas tellurium hexafluoride is poisonous, not very stable and can be hydrolyzed by water within a day . In contrast, the metal hexafluorides are corrosive, easily hydrolyzable and can react violently with water. Some of them can be used as fluorinating agents . The metal hexafluorides have a high electron affinity , which makes them strong oxidizing agents . Platinum hexafluoride is characterized by its ability to oxidize the oxygen molecule (O 2 ). It was therefore the first compound to be reacted with xenon (see xenon hexafluoroplatinate ).
use
Some of the metal hexafluorides find practical applications because of their volatility. Uranium hexafluoride is used for uranium enrichment in order to obtain fuel for nuclear reactors . The fluoride volatility is also used for the reprocessing of nuclear fuels . Tungsten hexafluoride is used in the chemical vapor deposition process in the manufacture of semiconductors .
More hexafluorides
The synthesis of polonium hexafluoride (PoF 6 ) was attempted in 1945, but did not lead to any clear results; the boiling point was estimated at −40 ° C. Ab initio and Dirac-Hartree-Fock calculations describe some properties of the not yet synthesized radon hexafluoride (RnF 6 ). Americium hexafluoride (AmF 6 ) could not be produced by direct fluorination of americium (IV) fluoride; the synthesis has not yet taken place in 1990 either.
literature
- NP Galkin, Yu N. Tumanov: "Reactivity and Thermal Stability of Hexafluorides", in: Russ. Chem. Rev. , 1971 , 40 (2), pp. 154-164 ( abstract ; doi : 10.1070 / RC1971v040n02ABEH001902 ).
Individual evidence
- ↑ Entry on sulfur hexafluoride in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Entry on selenium hexafluoride in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Entry on tellurium hexafluoride in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Gmelin's Handbook of Inorganic Chemistry , System No. 11, Tellurium, Part B 2, p. 26.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-98.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-77.
- ↑ a b c d e f g h i j k l m n o p q r s T. Drews, J. Supeł, A. Hagenbach, K. Seppelt: "Solid State Molecular Structures of Transition Metal Hexafluorides", in: Inorganic Chemistry , 2006 , 45 (9), pp. 3782-3788 ( doi : 10.1021 / ic052029f ; PMID 16634614 ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-93.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-86.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-85.
- ↑ Entry on tungsten hexafluoride in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-85.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-79.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-68.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-81.
- ↑ Entry on uranium hexafluoride in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Gmelin's Handbook of Inorganic Chemistry , System No. 55, Uranium, Part C 8, p. 97.
- ↑ a b c Masao Kimura, Werner Schomaker, Darwin W. Smith, Bernard Weinstock: "Electron-Diffraction Investigation of the Hexafluorides of Tungsten, Osmium, Iridium, Uranium, Neptunium, and Plutonium", in: J. Chem. Phys. , 1968 , 48 (8), pp. 4001-4012 ( doi : 10.1063 / 1.1669727 ).
- ↑ C. Keller: "The Chemistry of Neptunium", in: Fortschr. chem. Forsch. , 1969/70 , 13/1 , pp. 1–124, here: pp. 71–75 ( doi : 10.1007 / BFb0051170 ).
- ↑ Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurane, Part C, pp. 108-114.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-81.
- ↑ N. Bartlett: “The Oxidizing Properties of the Third Transition Series Hexafluorides and Related Compounds”, in: Angewandte Chemie International Edition in English , 1968 , 7 (6), pp. 433-439 ( doi : 10.1002 / anie.196804331 ) .
- ↑ "Tungsten and Tungsten Silicide Chemical Vapor Deposition" .
- ↑ Summary of work to date on volatile neutron source , Monsanto Chemical Company, Unit 3 abstracts of progress reports, August 16–31, 1945 ( abstract ; PDF ).
- ↑ Michael Filatov, Dieter Cremer: "Bonding in Radon Hexafluoride: An Unusual Relativistic Problem?", In: Phys. Chem. Chem. Phys. , 2003 , 5 , pp. 1103-1105 ( doi : 10.1039 / b212460m ).
- ↑ John G. Malm, Bernard Weinstock, E. Eugene Weaver: “The Preparation and Properties of NpF 6 ; a Comparison with PuF 6 “, in: J. Phys. Chem. , 1958 , 62 (12), pp. 1506-1508 ( doi : 10.1021 / j150570a009 ).
- ↑ KC Kim, RN Mulford: "Vibrational Properties of Actinide (U, Np, Pu, Am) Hexafluoride Molecules", in: Journal of Molecular Structure: THEOCHEM , 1990 , 207 (3-4), pp. 293-299 ( doi : 10.1016 / 0166-1280 (90) 85031-H ).