Plutonium (VI) fluoride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Crystal system | ||||||||||
| Space group |
Pnma (No. 62) |
|||||||||
| Lattice parameters |
a = 995 pm |
|||||||||
| General | ||||||||||
| Surname | Plutonium (VI) fluoride | |||||||||
| other names |
Plutonium hexafluoride |
|||||||||
| Molecular formula | PuF 6 | |||||||||
| Brief description |
red-brown crystalline solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 358.06 g mol −1 ( 244 Pu) | |||||||||
| Physical state |
firmly |
|||||||||
| density |
5.08 g cm −3 |
|||||||||
| Melting point |
52 ° C |
|||||||||
| boiling point |
62 ° C |
|||||||||
| Hazard and safety information | ||||||||||
 Radioactive |
||||||||||
|
||||||||||
| Thermodynamic properties | ||||||||||
| ΔH f 0 |
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Plutonium (VI) fluoride (PuF 6 ), usually called plutonium hexafluoride , is a chemical compound made up of the elements plutonium and fluorine . It is a red-brown crystalline solid that is highly volatile , highly radioactive and corrosive . Plutonium hexafluoride is stable in dry air, but reacts violently with water. In most cases it is obtained from plutonium (IV) fluoride (PuF 4 ) by reaction with elemental fluorine (F 2 ).
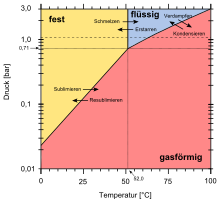
At normal pressure it melts at 52 ° C and boils at 62 ° C. It is the only plutonium compound that can easily be converted into the gas phase. Because of these properties, plutonium hexafluoride plays a role in the enrichment of plutonium, especially for the isolation of the pure isotope 239 Pu, as well as in the separation of uranium and plutonium in the processing of nuclear waste .
history
Very soon after the discovery and isolation of plutonium in 1940, the existence of plutonium hexafluoride was postulated. Early experiments, specifically designed to produce plutonium hexafluoride analogous to uranium hexafluoride , produced contradicting results. The proposal that uranium could be separated from uranium from plutonium by fluorination was published as early as June 1942. Due to the ongoing Second World War , the other works were initially kept under lock and key and not published.
Experiments carried out at the time, which were carried out with very small amounts of plutonium, showed that volatilization of plutonium in a flow of fluorine only occurred above 700 ° C. Later experiments showed that plutonium could be volatilized from a copper plate by fluorine at 500 ° C and that the rate of volatilization in fluorine decreased from uranium to neptunium to plutonium - i.e. with increasing atomic number . Brown and Hill, who had plutonium in a few micrograms available, concluded in 1942 from distillation experiments with uranium hexafluoride that higher fluorides of plutonium would be unstable and that they would decompose again to plutonium tetrafluoride at room temperature . They also concluded that the vapor pressure of this plutonium compound corresponds to that of uranium hexafluoride. Davidson, Katz and Orlemann found in 1943 that plutonium evaporated from a nickel vessel under a fluorine atmosphere and the product was deposited on a platinum surface. In 1944, Fisher, Vaslow and Tevebaugh hypothesized that the enthalpy of formation of the higher fluorides is positive , i.e. the formation reaction is endothermic , and that these should therefore only be stable at higher temperatures in a fluorine atmosphere. Florin made a volatile compound of plutonium in 1944, which was believed to be plutonium hexafluoride. However, the product decomposed before it could be identified. The volatile material was initially deposited on glass in a cooler place, after a few minutes the volatility decreased noticeably and the material changed its physical state from solid to liquid. It was assumed that the fluoride reacted with the glass surface. To predict the properties of plutonium hexafluoride, Brewer, Bromley, Gilles, and Lofgren used known thermodynamic data and other information derived from comparing uranium and plutonium compounds.
Florin delivered the main results in 1950. More precise thermodynamic data and technical improvements to the equipment used were developed in 1952 and summarized in 1953. Four British studies followed in the years 1951 to 1953. Malm and Weinstock as well as Hawkins, Mattraw and Sabol contributed further results. The previous publication, which was previously under lock and key, was released until 1955 and published jointly in the Journal of Inorganic and Nuclear Chemistry in 1956. All available data up to May 1963 have been summarized by Steindler.
presentation
Standard method
The common method for the preparation of plutonium hexafluoride is the conversion of plutonium (IV) fluoride (PuF 4 ) with elemental fluorine (F 2 ).
The reaction is endothermic . The product forms relatively quickly at temperatures of 750 ° C; high yields can be achieved if the PuF 6 condenses quickly and is thus removed from the equilibrium .
In the same way, uranium hexafluoride (UF 6 ) is produced relatively quickly at 300 ° C from uranium tetrafluoride (UF 4 ) and fluorine (F 2 ), neptunium hexafluoride (NpF 6 ) at 500 ° C from neptunium tetrafluoride (NpF 4 ) and F 2 .
Other methods
Plutonium hexafluoride has also been produced by fluorinating plutonium (III) fluoride or plutonium (IV) oxide .
Plutonium (III) fluoride, plutonium (IV) oxalate and plutonium (IV) oxide were each reacted in a gas stream of hydrogen fluoride and oxygen at about 700 ° C .; In all cases this led to a complete volatilization of these starting materials to form plutonium hexafluoride.
Plutonium tetrafluoride was also converted to plutonium hexafluoride with oxygen at 800 ° C.
In contrast, the synthesis succeeds even at room or lower temperatures using krypton difluoride (KrF 2 ) or dioxygen difluoride (O 2 F 2 ) as well as by irradiation with UV light .
properties
Physical Properties
Plutonium hexafluoride, freshly condensed at −180 ° C and sealed in a vacuum, is initially a colorless crystalline substance, similar to uranium hexafluoride; when warming to room temperature, the color changes to red-brown. Under normal pressure (1,013.25 hPa ) it melts at 52 ° C and boils at 62 ° C. The triple point at which the three phases solid, liquid and gaseous are in equilibrium is at a temperature of 51.58 ° C (324.74 K) at a pressure of 710 hPa (533 Torr). This means that below this pressure solid plutonium hexafluoride changes directly into the gaseous state by sublimation when heated . The volatility of PuF 6 is similar to that of uranium hexafluoride (UF 6 ) and neptunium hexafluoride (NpF 6 ); together they belong to the three previously known hexafluorides of the actinide elements . The Bildungsentropie (S 0 m ) is fixed for PuF 6 : 221.8 ± 1.1 J · K -1 · mol -1 , gaseous PuF 6 : 368.9 ± 1.0 J · K -1 · mol - 1 . Solid PuF 6 is paramagnetic ; the molar magnetic susceptibility χ mol is 173 · 10 −6 cm 3 · mol −1 (22 ° C).
Crystal and molecular structure
Plutonium hexafluoride is a covalent compound and not a salt. It crystallizes in the orthorhombic crystal system in the space group Pnma (No. 62) with the lattice parameters a = 995 pm , b = 902.0 pm and c = 526.0 pm with four formula units per unit cell . In the gaseous state it consists of regular octahedral molecules ( O h ) with a uniform Pu – F bond length of 197.1 pm.
Spectroscopic properties
Plutonium hexafluoride has six fundamental vibrations . ν 1 , ν 2 and ν 3 are stretching vibrations and ν 4 , ν 5 and ν 6 are bending vibrations . ν 1 , ν 2 and ν 5 are Raman active , ν 3 and ν 4 are IR active , ν 6 is IR and Raman inactive. The Raman spectrum of PuF 6 could not yet be observed due to the rapid photochemical decomposition at 564.1 nm . If PuF 6 gas is excited at 532 nm, fluorescence in the 1900 and 4800 nm range can be observed; if 242 PuF 6 gas is excited at 1064 nm, a fluorescence maximum can be observed at 2300 nm.
Fundamental vibration ν 1 ν 2 ν 3 ν 4 ν 5 ν 6 Term symbol A 1g E g F 1u F 1u F 2g F 2u Wave number (cm −1 ) 628 523 615 203 211 171 IR active - - + + - - Raman active + + - - + -
Chemical properties
Reactions with other substances
Plutonium hexafluoride is stable in dry air. On the other hand, it reacts very violently with water (due to humidity in the air), producing the water-soluble plutonyl (VI) fluoride (PuO 2 F 2 ) and hydrogen fluoride (HF).
It can be stored for a very long time at room temperature in quartz or PYREX ampoules if it is ensured that there are no traces of moisture, the glass itself is free of all gas inclusions and any hydrogen fluoride (HF) that may be present has been completely removed.
The most complete possible reduction of plutonium hexafluoride to plutonium dioxide is also important. The carbon monoxide produced in an oxygen-methane flame is a good reducing agent for obtaining the actinide dioxides directly from the hexafluorides.
Decomposition reactions
Plutonium hexafluoride in turn decomposes to plutonium (IV) fluoride (PuF 4 ) and elemental fluorine (F 2 ):
- A noticeable thermal decomposition to plutonium (IV) fluoride can be observed; it does not yet set in at room temperature, but takes place very quickly at 280.degree.
- It is also subject to autoradiolysis , i.e. decomposition by its own radioactivity , and also forms plutonium (IV) fluoride and elemental fluorine. As soon as emitted α-particles move through the crystal lattice, bonds are broken and the decomposition of PuF 6 to F 2 and the lower plutonium fluorides begins. The decomposition rate due to α radiation in the isotope 239 Pu averages 1.5% per day in the solid phase. The initial high rate of degradation of 1.78% per day can partly be attributed to the reaction of the PuF 6 with the container material. However, it is significantly smaller in the gas phase. If the pressure of the PuF 6 is reduced from 100 to 50 Torr (≈ 133 or 67 mbar ), a lower rate of degradation is observed. A surprisingly low gas pressure was measured in decades-old, tight PuF 6 cylinders; it was assumed that recombination with F atoms takes place. Plutonium hexafluoride is also decomposed by γ radiation .
- Both PuF 6 and NpF 6 are photosensitive and decompose to the tetrafluorides and fluorine. By laser irradiation with a wavelength of 337 nm ( nitrogen laser ) it decomposes to plutonium (V) fluoride (PuF 5 ) and fluorine. This laser-induced decomposition could be observed with decreasing efficiency up to a wavelength of 520 nm. An absorption band at 564.1 nm also leads to rapid photochemical decomposition in this area.
use
Plutonium hexafluoride plays a role in the enrichment of plutonium, especially in isolating the fissile isotope 239 Pu ( half-life : 24,110 years) from irradiated uranium.
Above all, when used for nuclear weapons, the 241 Pu present as an impurity (half-life: 14.35 years) must be removed for two reasons:
- It generates enough neutrons through spontaneous fission to trigger an uncontrolled premature ignition.
- It also breaks down to 241 Am due to β-decay , so that after a long period of storage noticeable amounts of americium are produced which have to be removed.
The separation of plutonium and the americium contained therein is achieved by reaction with dioxygen difluoride (O 2 F 2 ). PuF 4 that has been stored for a long time is fluorinated at room temperature in order to obtain PuF 6 gas, which is collected separately and reduced again to PuF 4 . The americium (IV) fluoride (AmF 4 ) contained is not converted. The reaction product therefore contains very little americium, while the unreacted solid residue has correspondingly increased concentrations of americium.
The separation of uranium and plutonium hexafluoride are current issues in the processing of nuclear waste . From molten salts containing uranium and plutonium, the uranium can largely be removed as UF 6 by fluorination , since it is more stable at high temperatures; In contrast, only a small amount of plutonium escapes as PuF 6 .
safety instructions
Plutonium hexafluoride acts on the human body in three main ways:
- It is a very aggressive substance that attacks any tissue. When the gas comes into contact with body fluids, hydrofluoric acid is formed , which causes chemical burns on the skin and the mucous membranes of the respiratory tract. Human exposure to the gas initially affects the eyes and respiratory tract, causing irritation, loss of vision, coughing, and excessive saliva and sputum formation. After prolonged exposure, this leads to pneumonitis and pulmonary edema and can lead to death.
- It is very toxic when inhaled and swallowed. In addition, there is a risk of accumulation in the human body, especially in the liver and kidneys.
- It is very radioactive .
literature
- Martin J. Steindler: Laboratory Investigations in Support of Fluid-bed Fluoride Volatility Processes, Part II, The Properties of Plutonium Hexafluoride (Argonne National Laboratory Report ANL-6753); August 1, 1963 ( doi : 10.2172 / 4170539 ; abstract ; PDF ).
- David L. Clark, Siegfried S. Hecker, Gordon D. Jarvinen, Mary P. Neu: Plutonium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 813-1264 ( doi : 10.1007 / 1-4020-3598-5_7 ).
- RC Burns, TA O'Donnell, CH Randall: Reactivity of Transition Metal Fluorides - XII: Plutonium Hexafluoride , in: Journal of Inorganic and Nuclear Chemistry , 1981 , 43 (6), pp. 1231-1238 ( doi : 10.1016 / 0022- 1902 (81) 80023-1 ).
Individual evidence
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-81.
- ↑ a b c d e Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurane, Part C, pp. 108–114.
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ a b c David L. Clark, Siegfried S. Hecker, Gordon D. Jarvinen, Mary P. New: Plutonium , p. 1087.
- ^ GT Seaborg; University of Chicago Metallurgical Laboratory Report CN-125 (1942).
- ^ A b c d Martin J. Steindler: Laboratory Investigations in Support of Fluid-bed Fluoride Volatility Processes, Part II, The Properties of Plutonium Hexafluoride (Argonne National Laboratory Report ANL-6753); August 1, 1963 ( doi : 10.2172 / 4170539 ; abstract ; PDF ).
- ^ HS Brown, OF Hill, AH Jaffay; University of Chicago Metallurgical Laboratory Report CN-343 (1942).
- ^ HS Brown, OF Hill; University of Chicago Metallurgical Laboratory Report CN-363 (November 12, 1942).
- ^ NR Davidson, JJ Katz, OF Orlemann; University of Chicago Metallurgical Laboratory Report CN-987 (October 11, 1943).
- ↑ RW Fisher, F. Vaslow, AD Tevebaugh; Iowa State College, Report CN-1783 (August 10, 1944).
- ↑ AE Florin ; University of Chicago Metallurgical Laboratory Report CN-2159 (October 1, 1944).
- ↑ L. Brewer, L. Bromley, PW Gilles, NL Lofgren; University of California Radiation Laboratory Report CN-3300 (October 10, 1945).
- ↑ L. Brewer, L. Bromley, PW Gilles, NL Lofgren; University of California Radiation Laboratory Report CN-3378 (December 1, 1945).
- ↑ L. Brewer, LA Bromley, PW Gilles, NL Lofgren: The Higher Fluorides of Plutonium (University of California Radiation Laboratory Report UCRL-633); March 20, 1950 ( abstract ).
- ^ Alan E. Florin: Plutonium Hexafluoride, Plutonium (VI) Oxyfluoride: Preparation, Identification, and Some Properties (Los Alamos Scientific Laboratory Reports LAMS-1118); October 16, 1950 ( PDF ; 1.1 MB).
- ^ AE Florin: Plutonium Hexafluoride: Second Report On The Preparation and Properties (Los Alamos Scientific Laboratory Reports LA-1168); November 9, 1950 ( PDF ; 827 kB).
- ^ AE Florin: Thermodynamic Properties of Plutonium Hexafluoride: a Preliminary Report (Los Alamos Scientific Laboratory Reports LAMS-1587); May 15, 1953 ( PDF ; 515 kB).
- ^ IR Tannenbaum, AE Florin: An Improved Apparatus for the Preparation of Plutonium Hexafluoride (Los Alamos Scientific Laboratory Reports LA-1580); May 15, 1953 ( PDF ; 582 kB).
- ^ A b J.K. Dawson, AE Truswell: The Preparation of Plutonium Trifluoride and Tetrafluoride by the Use of Hydrogen Fluoride ( Atomic Energy Research Establishment Report AERE C / R-662); February 22, 1951 ( abstract ).
- ↑ a b C. J. Mandleberg, et al. (Atomic Energy Research Establishment Report AERE C / R-157); 1952 ( abstract ).
- ↑ CJ Mandleberg, HK Rae, R. Hurst, G. Long, D. Davis, KE Francis: Plutonium Hexafluoride, Part I, Preparation and Some Physical Properties (Atomic Energy Research Establishment Report AERE C / R-1172); April 1953.
- ↑ R. Hurst, CJ Mandleberg, HK Rae, D. Davis, KE Francis: plutonium hexafluorides, Part II, Preparation and Some Physical Properties (Atomic Energy Research Establishment Report AERE C / R-1312); January 1953.
- ^ JG Malm, B. Weinstock: Argonne Plutonium Hexafluoride Program (Argonne National Laboratory Report ANL-5366); September 1, 1954 ( abstract ).
- ↑ a b c N.J. Hawkins, HC Mattraw, WW Sabol: Infrared Spectrum and Thermodynamic Properties of PuF 6 (Knoll's Atomic Power Laboratory Report KAPL-1007); May 24, 1954 ( abstract ).
- ↑ a b c d C. J. Mandleberg, HK Rae, R. Hurst, G. Long, D. Davies, KE Francis: Plutonium Hexafluoride , in: Journal of Inorganic and Nuclear Chemistry , 1956 , 2 (5-6), p. 358 -367 ( doi : 10.1016 / 0022-1902 (56) 80090-0 ).
- ↑ a b c Alan E. Florin, Irving R. Tannenbaum, Joe F. Lemons: Preparation and Properties of Plutonium Hexafluoride and Identification of Plutonium (VI) Oxyfluoride , in: Journal of Inorganic and Nuclear Chemistry , 1956 , 2 (5-6 ), Pp. 368-379 ( doi : 10.1016 / 0022-1902 (56) 80091-2 ).
- ↑ a b c d e Bernard Weinstock, John G. Malm: The Properties of Plutonium Hexafluoride , in: Journal of Inorganic and Nuclear Chemistry , 1956 , 2 (5–6), pp. 380–394 ( doi : 10.1016 / 0022- 1902 (56) 80092-4 ).
- ^ MJ Steindler, DV Steidl, RK Steunenberg: The Fluorination of Plutonium Tetrafluoride (Argonne National Laboratory Report ANL-5875); June 1, 1958 ( abstract ).
- ^ MJ Steindler, DV Steidl, RK Steunenberg: The Fluorination of Plutonium Tetrafluoride and Plutonium Dioxide by Fluorine , in: Nucl. Sci. and Eng. , 1959 , 6 (4), pp. 333-340 ( abstract ).
- ^ A b c John G. Malm, Bernard Weinstock, E. Eugene Weaver: The Preparation and Properties of NpF 6 ; a Comparison with PuF 6 , in: J. Phys. Chem. , 1958 , 62 (12), pp. 1506-1508 ( doi : 10.1021 / j150570a009 ).
- ↑ LB Asprey, PG Eller, Scott A. Kinkead: Formation of Actinide Hexafluorides at Ambient Temperatures with Krypton Difluoride , in: Inorg. Chem. , 1986 , 25 (5), pp. 670-672 ( doi : 10.1021 / ic00225a016 ).
- ↑ JG Malm, PG Eller, LB Asprey: Low Temperature Synthesis of Plutonium Hexafluoride Using Dioxygen Difluoride , in: J. Am. Chem. Soc. , 1984 , 106 (9), pp. 2726-2727 ( doi : 10.1021 / ja00321a056 ).
- ↑ PE Erilov, VV Titov, VF Serik, VB Sokolov: Low-Temperature Synthesis of Plutonium Hexafluoride , in: Atomic Energy , 2002 , 92 (1), pp. 57-63 ( doi : 10.1023 / A: 1015106730457 ).
- ↑ LE Trevorrow, TJ Gerding, MJ Steindler: Ultraviolet-Activated Synthesis of Plutonium Hexafluoride at Room Temperature , in: Inorganic and Nuclear Chemistry Letters , 1969 , 5 (10), pp. 837-839 ( doi : 10.1016 / 0020-1650 ( 69) 80068-1 ; abstract ).
- ↑ a b D. M. Gruen, JG Malm, B. Weinstock: Magnetic Susceptibility of Plutonium Hexafluoride , in: J. Chem. Phys. , 1956 , 24 (4), pp. 905-906 ( doi : 10.1063 / 1.1742635 ).
- ^ A b Masao Kimura, Werner Schomaker, Darwin W. Smith, Bernard Weinstock: Electron-Diffraction Investigation of the Hexafluorides of Tungsten, Osmium, Iridium, Uranium, Neptunium, and Plutonium , in: J. Chem. Phys. , 1968 , 48 (8), pp. 4001-4012 ( doi : 10.1063 / 1.1669727 ).
- ^ Lester R. Morss, Norman M. Edelstein, Jean Fuger: The Chemistry of the Actinide and Transactinide Elements. Volumes 1-6. Springer, 2010, ISBN 978-94-007-0210-3 , pp. 1086, 1090.
- ↑ Martin J. Steindler, William H. Gunther: The Absorption Spectrum of Plutonium Hexafluoride , in: Spectrochimica Acta , 1964 , 20 (8), pp. 1319-1322 ( doi : 10.1016 / 0371-1951 (64) 80159-4 ) .
- ^ R. Kugel, C. Williams, M. Fred, JG Malm, WT Carnall, JC Hindman, WJ Childs, LS Goodman: Isotope Effects in the Molecular Spectrum of Plutonium Hexafluoride , in: J. Chem. Phys. , 1976 , 65 (9), pp. 3486-3492 ( doi : 10.1063 / 1.433575 ).
- ↑ RT Walters, RA Briesmeister: Absorption Spectrum of Plutonium Hexafluoride in the 3000–9000 Å Spectral Region , in: Spectrochimica Acta Part A: Molecular Spectroscopy , 1984 , 40 (7), pp. 587–589 ( doi : 10.1016 / 0584- 8539 (84) 80108-7 ).
- ↑ B. Weinstock, EE Weaver, JG Malm: Vapor Pressures of NpF 6 and PuF 6 ; Thermodynamic Calculations with UF 6 , NpF 6 and PuF 6 , in: Journal of Inorganic and Nuclear Chemistry , 1959 , 11 (2), pp. 104-114 ( doi : 10.1016 / 0022-1902 (59) 80054-3 ).
- ↑ KC Kim, RN Mulford: Vibrational Properties of Actinide (U, Np, Pu, Am) Hexafluoride Molecules , in: Journal of Molecular Structure: THEOCHEM , 1990 , 207 (3-4), pp. 293-299 ( doi : 10.1016 / 0166-1280 (90) 85031-H ).
- ↑ James V. Beitz, Clayton W. Williams, WT Carnall: Fluorescence Studies of Neptunium and Plutonium Hexafluoride Vapors , in: J. Chem. Phys. , 1982 , 76 , pp. 2756-2757 ( doi : 10.1063 / 1.443223 ).
- ↑ James V. Beitz, Clayton W. Williams, WT Carnall: Plutonium Hexafluoride Gas Photophysics and Photochemistry , in: Plutonium Chemistry , ACS Symposium Series 1983, Vol. 216, Chapter 11, ISBN 0-8412-0772-0 , p. 155 -172 ( doi : 10.1021 / bk-1983-0216.ch011 ).
- ↑ NJ Hawkins, HC Mattraw, WW Sabol: Infrared Spectrum of plutonium hexafluoride , in: J. Chem. Phys. , 1955 , 23 , pp. 2191-2192 ( doi : 10.1063 / 1.1740699 ).
- ↑ John G. Malm, Bernard Weinstock, Howard H. Claassen: Infrared Spectra of NpF 6 and PuF 6 , in: J. Chem. Phys. , 1955 , 23 , pp. 2192-2193 ( doi : 10.1063 / 1.1740700 ).
- ↑ RW Kessie: Plutonium and Uranium Hexafluoride Hydrolysis Kinetics , in: Ind. Eng. Chem. Proc. Of. Dev. , 1967 , 6 (1), pp. 105-111 ( doi : 10.1021 / i260021a018 ).
- ↑ AM Pokidyshev, IA Tsarenko, VF Serik, VB Sokolov: Reduction of Plutonium Hexafluoride Using Gaseous Reagents , in: Atomic Energy , 2003 , 95 (4), pp. 701-708 ( doi : 10.1023 / B: ATEN.0000010988.94533.24 ).
- ↑ LE Trevorrow, WA Shinn, RK Steunenberg: The Thermal Decomposition of Plutonium Hexafluoride , in: J. Phys. Chem. , 1961 , 65 (3), pp. 398-403 ( doi : 10.1021 / j100821a003 ).
- Jump up ↑ J. Fischer, L. Trevorrow, W. Shinn: The Kinetics and Mechanism of the Thermal Decomposition of Plutonium Hexafluoride , in: J. Phys. Chem. , 1961 , 65 (10), pp. 1843-1846 ( doi : 10.1021 / j100827a036 ).
- ↑ Jack Fischer, LE Trevorrow, GJ Vogel, WA Shinn: Plutonium Hexafluoride Thermal Decomposition Rates , in: Ind. Eng. Chem. Proc. Of. Dev. , 1962 , 1 (1), pp. 47-51 ( doi : 10.1021 / i260001a010 ).
- ↑ S. Tsujimura, K. Hirano, A. Takahashi, G. Fujisawa: Thermal Decomposition of Plutonium Hexafluoride , in: J. Nucl. Sci. and Tech. , 1972 , 9 , pp. 534-537 ( doi : 10.3327 / jnst.9.534 ; PDF ).
- ^ RP Wagner, WA Shinn, J. Fischer, MJ Steindler: Laboratory Investigations in Support of Fluid-bed Fluoride Volatility Processes, Part VII, The Decomposition of Gaseous Plutonium Hexafluoride by Alpha Radiation (Argonne National Laboratory Report ANL-7013); May 1, 1965 ( doi : 10.2172 / 4628896 ; abstract ; PDF ).
- ^ Ned E. Bibler: α and β Radiolysis of Plutonium Hexafluoride Vapor , in: J. Phys. Chem. , 1979 , 83 (17), pp. 2179-2186 ( doi : 10.1021 / j100480a001 ).
- ↑ LR Morss: PuF 6 gas pressure in cylinders aged , personal communication, DL Clark, Los Alamos, NM (2005).
- ↑ MJ Steindler, DV Steidl, J. Fischer: The Decomposition of Plutonium Hexafluoride by Gamma Radiation , in: Journal of Inorganic and Nuclear Chemistry , 1964 , 26 (11), pp. 1869–1878 ( doi : 10.1016 / 0022-1902 ( 64) 80011-7 ).
- ↑ US Patent 4670239: Photochemical Preparation of Plutonium Pentafluoride , June 2, 1987 ( PDF ).
- ^ A b G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties. In: Nuclear Physics. Volume A 729, 2003, pp. 3–128, here: pp. 118–119. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ TR Mills, LW Reese: Separation of Plutonium and Americium by Low-Temperature Fluorination , in: Journal of Alloys and Compounds , 1994 , 213-214 , pp. 360-362 ( doi : 10.1016 / 0925-8388 (94) 90931- 8 ).
- ↑ US Patent 3708568: Removal of Plutonium from Plutonium Hexafluoride-Uranium Hexafluoride Mixtures , January 2, 1973 ( PDF ).
- ↑ US Patent 4172114: Method for Purifying Plutonium Hexafluoride , October 23, 1979 ( PDF ).
- ^ W. Scott Moser, James D. Navratil: Review of Major Plutonium Pyrochemical Technology , in: Journal of the Less Common Metals , 1984 , 100 , pp. 171-187 ( doi : 10.1016 / 0022-5088 (84) 90062-6 ).
- ↑ Yu. V. Drobyshevskii, VK Ezhov, EA Lobikov, VN Prusakov, VF Serik, VB Sokolov: Application of Physical Methods for Reducing Plutonium Hexafluoride , in: Atomic Energy , 2002 , 93 (1), pp. 578-588 ( doi : 10.1023 / A: 1020840716387 ).
- ^ Evaluation of the US Department of Energy's Alternatives for the Removal and Disposition of Molten Salt Reactor Experiment Fluoride Salts. Retrieved May 3, 2010 .