Technetium (VI) fluoride
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
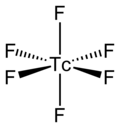
|
|||||||
| General | |||||||
| Surname | Technetium (VI) fluoride | ||||||
| other names |
Technetium hexafluoride |
||||||
| Molecular formula | TcF 6 | ||||||
| Brief description |
golden yellow crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 212 g mol −1 ( 98 Tc) | ||||||
| Physical state |
firmly |
||||||
| density |
3.58 g cm −3 (−140 ° C) |
||||||
| Melting point |
37.4 ° C |
||||||
| boiling point |
55.3 ° C |
||||||
| Hazard and safety information | |||||||
 Radioactive |
|||||||
|
|||||||
| Thermodynamic properties | |||||||
| ΔH f 0 |
4616.84 ± 1.60 J mol −1 |
||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Technetium (VI) fluoride (TcF 6 ), usually also technetium hexafluoride , is an inorganic compound of the elements technetium and fluorine and belongs to the group of hexafluorides . It is a golden yellow crystalline solid with a low melting and boiling point. In this compound technetium has the oxidation state +6, the highest of the technetium halides. The other hexavalent compound is technetium (VI) chloride , TcCl 6 . In this respect, technetium differs from rhenium, which in its highest oxidation state forms the heptafluoride ReF 7 . Since technetium is a fission product of uranium , technetium hexafluoride occurs as an impurity in uranium hexafluoride .
presentation
Technetium hexafluoride is produced by direct conversion of the metal in an excess of elemental fluorine (F 2 ) at 400 ° C.
properties
Physical Properties
Technetium hexafluoride is a golden yellow crystalline solid that melts at 37.4 ° C and boils at 55.3 ° C. A solid phase transition can be observed at −4.54 ° C. Above this temperature (measured at 10 ° C) it crystallizes in the cubic crystal system with the lattice parameter a = 616 pm and two formula units per unit cell with a calculated density of 3.02 g · cm −3 . Below this temperature (measured at −19 ° C) it crystallizes in the orthorhombic crystal system in the space group Pnma (No. 62) with the lattice parameters a = 955 pm, b = 874 pm and c = 502 pm and four formula units per unit cell with a calculated one Density of 3.38 g cm −3 . The lattice parameters of the orthorhombic phase at −140 ° C are: a = 936.0 pm, b = 851.7 pm and c = 493.4 pm with a calculated density of 3.58 g cm −3 .
Infrared and Raman spectroscopy show that the TcF 6 molecule is octahedral ( O h ); the Tc – F bond length is 181.2 pm. Measurements of the magnetic moment give a value of 0.45 μ B .
Chemical properties
TcF 6 reacts quantitatively to TcF 5 with iodine in iodine pentafluoride solutions . It reacts with alkali metal chlorides in iodine pentafluoride solution to form hexafluorotechnetates (TcF 6 - ). TcF 6 disproportionates during hydrolysis with aqueous NaOH and forms a black precipitate of technetium (IV) oxide TcO 2 , which dissolves when hydrogen peroxide H 2 O 2 is added . In hydrogen fluoride solution, TcF 6 reacts with hydrazinium fluoride to form N 2 H 6 TcF 6 or N 2 H 6 (TcF 6 ) 2 .
Individual evidence
- ↑ a b c H. Selig, CL Chernick, JG Malm: "The Preparation and Properties of TcF 6 ", in: J. Inorg. Nucl. Chem. , 1961 , 19 (3-4), pp. 377-381; doi : 10.1016 / 0022-1902 (61) 80132-2 .
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-93.
- ↑ a b c T. Drews, J. Supeł, A. Hagenbach, K. Seppelt: "Solid State Molecular Structures of Transition Metal Hexafluorides", in: Inorganic Chemistry , 2006 , 45 (9), pp. 3782-3788; doi : 10.1021 / ic052029f ; PMID 16634614 .
- ↑ a b c H. Selig, JG Malm: "The Vapor-Pressure and Transition Points of TcF 6 ", in: J. Inorg. Nucl. Chem. , 1962 , 24 (6), pp. 641-644; doi : 10.1016 / 0022-1902 (62) 80082-7 .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ Darrell W. Osborne, Felix Schreiner, Klaus Otto, John G. Malm, Henry Selig: “Heat capacity, entropy, and Gibbs energy of technetium hexafluoride between 2.23 and 350 K; magnetic anomaly at 3.12 K; mean β energy of 99 Tc ", in: Journal of Chemical Physics , 1978 , 68 (3), pp. 1108-1118; doi : 10.1063 / 1.435789 .
- ^ Stanley Siegel, David A. Northrop: "X-Ray Diffraction Studies of Some Transition Metal Hexafluorides", in: Inorg. Chem. , 1966 , 5 (12), pp. 2187-2188; doi : 10.1021 / ic50046a025
- ^ Howard H. Claassen, Henry Selig, John G. Malm: "Vibrational Spectra of MoF 6 and TcF 6 ", in: Journal of Chemical Physics , 1962 , 36 (11), pp. 2888-2890; doi : 10.1063 / 1.1732396 .
- ^ Howard H. Claassen, Gordon L. Goodman, John H. Holloway, Henry Selig: "Raman Spectra of MoF 6 , TcF 6 , ReF 6 , UF 6 , SF 6 , SeF 6 , and TeF 6 in the Vapor State", in: Journal of Chemical Physics , 1970 , 53 (1), pp. 341-348; doi : 10.1063 / 1.1673786 .
- ^ Henry Selig, Fred A. Cafasso, Dieter M. Gruen, John G. Malm: "Magnetic Susceptibility of ReF 6 ", in: Journal of Chemical Physics , 1962 , 36 (12), pp. 3440-3444; doi : 10.1063 / 1.1732477 .
- ↑ J. Binenboym, H. Selig: J. Inorg. Nucl Chem. Suppl. , 1976 , pp. 231-232.
- ^ AJ Edwards, D. Hugill, RD Peacock: "New Fluorine Compounds of Technetium", in: Nature , 1963 , 200 , p. 672; doi : 10.1038 / 200672a0 .
- ↑ D. Hugill, RD Peacock: “Some quinquevalent fluorotechnetates”, in: J. Chem. Soc. A , 1966 , pp. 1339-1341; doi : 10.1039 / J19660001339 .
- ↑ Boris Frlec, Henry Selig, Herbert H. Hyman: "Hydrazinium (+2) Hexafluorometalates (IV) and - (V) in the 4d and 5d Transition Series", in: Inorg. Chem. , 1967 , 6 (10), pp. 1775-1783; doi : 10.1021 / ic50056a004 .
literature
- Gmelin's Handbook of Inorganic Chemistry , System No. 69, Technetium, Supplement Volume 2, pp. 79-83.
