Rhenium (VII) fluoride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Re 7+ __ F - | ||||||||||
| General | ||||||||||
| Surname | Rhenium (VII) fluoride | |||||||||
| other names |
Rhenium heptafluoride |
|||||||||
| Ratio formula | ReF 7 | |||||||||
| Brief description |
yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 319.20 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
4.3 g cm −3 |
|||||||||
| Melting point |
48.3 ° C |
|||||||||
| boiling point |
73.7 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Rhenium (VII) fluoride , usually also called rhenium heptafluoride , is a chemical compound of the elements rhenium and fluorine and belongs to the fluoride group . It is the only known thermally stable metal heptafluoride.
Extraction and presentation
Rhenium (VII) fluoride is produced by reacting the elements at 400 ° C.
properties
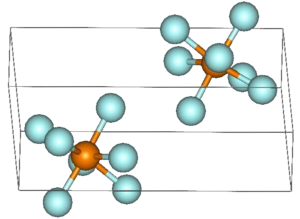
Rhenium (VII) fluoride is a yellow solid that melts at low temperatures. It has a distorted pentagonal bipyramidal structure similar to that of iodine heptafluoride . Below the phase transition at 153 K , the crystal lies in the triclinic space group space group C 1 (space group no. 2, position 3) with the lattice constants a = 5.5039 Å , b = 8.5026 Å , c = 9.0916 Å , α = 88.512 °, β = 93.842 ° and γ = 89.496 ° in front. At higher temperatures the crystal changes into a cubic structure with the space group space group Im 3 m (space group no. 229) with a lattice constant a = 6.2027 Å .
Individual evidence
- ↑ a b c d WebElements: Rhenium heptafluoride
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Greenwood, Norman N .; Earnshaw, Alan. (1997), Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann, ISBN 0-08037941-9 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1625.
- ↑ The numbering of this axis position does not correspond to the order of the International Tables for Crystallography , because it is not listed there.
- ^ Vogt T., Fitch AN, Cockcroft JK: Crystal and Molecular Structures of Rhenium Heptafluoride . In: Science . 263, No. 5151, 1994, p. 1265. doi : 10.1126 / science.263.5151.1265 . PMID 17817431 .
