Rhenium (VI) fluoride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
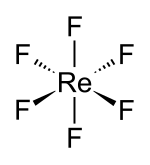
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Rhenium (VI) fluoride | |||||||||||||||
| other names |
Rhenium hexafluoride |
|||||||||||||||
| Molecular formula | ReF 6 | |||||||||||||||
| Brief description |
yellow crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 300.20 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
4.94 g cm −3 (−140 ° C) |
|||||||||||||||
| Melting point |
18.5 ° C |
|||||||||||||||
| boiling point |
33.7 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rhenium (VI) fluoride (ReF 6 ), usually also rhenium hexafluoride , is a chemical compound of the elements rhenium and fluorine and belongs to the group of hexafluorides .
presentation
Rhenium hexafluoride is produced by reacting rhenium (VII) fluoride ReF 7 with rhenium metal in an autoclave at 300 ° C.
The representation from the elements is also possible.
properties
At room temperature, rhenium hexafluoride is a liquid that solidifies to a yellow crystalline solid at 18.5 ° C or boils at 33.7 ° C. It crystallizes in the orthorhombic crystal system (measured at −140 ° C) in the space group Pnma (No. 62) with the lattice parameters a = 941.7 pm , b = 857.0 pm and c = 496.5 pm and four formula units per unit cell with a calculated density of 4.94 g cm −3 . The ReF 6 molecule is octahedral ( O h ); the Re – F bond length is 182.3 pm.
Individual evidence
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-85.
- ↑ a b c d T. Drews, J. Supeł, A. Hagenbach, K. Seppelt: "Solid State Molecular Structures of Transition Metal Hexafluorides", in: Inorganic Chemistry , 2006 , 45 (9), pp. 3782-3788; doi : 10.1021 / ic052029f ; PMID 16634614 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 271.
literature
- Gmelin's Handbook of Inorganic Chemistry , System No. 70, Rhenium, Part A, pp. 102-105.

