Sucrose
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| Crystal system |
monoclinic -sphenoid |
|||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Sucrose | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 12 H 22 O 11 | |||||||||||||||||||||
| Brief description |
colorless and odorless crystalline solid with a sweet taste |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 342.30 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.57 g cm −3 (30 ° C) |
|||||||||||||||||||||
| Melting point |
185–186 ° C (decomp. From approx. 160 ° C) |
|||||||||||||||||||||
| solubility |
very easily soluble in water (4.87 g per g water at 100 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
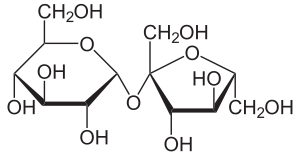
Sucrose [ zaxaroːzə ] (to Latin saccharum or ancient Greek σάκχαρον sákcharon , "sugar"), commonly known as table sugar , granulated sugar or simple sugar called, is a disaccharide and carbohydrate . Other names for sucrose are cane sugar, beet sugar, refined sugar or refined sugar, brown sugar (in the caramelized refined state), raw sugar (in the unrefined state , which is often brown, but not to be confused with it ) and sucrose or sucrose.
Sugar beet , sugar cane and sugar palm in particular contain this disaccharide in economically useful quantities. In sucrose, one molecule each of α- D - glucose and β- D - fructose are linked by an α, β-1,2- glycosidic bond .
The constitution was enlightened by Walter Norman Haworth .
Occurrence, extraction and importance in plants

Sucrose is formed by many plants through photosynthesis ; for the production of household sugar , sugar beet , sugar cane and sugar palm (mainly in Indonesia) are of particular importance. Sucrose is also obtained in smaller quantities from the sap of the sugar maple . In addition, the exclusively or predominantly sucrose-containing forms phloem sap of many plants the basis of the honey production - by the bees either directly vegetable exudates such as nectar or the honeydew precipitates referred to by phloem sap sucking insects (v a.. Schnabelkerfen such as aphids , scale insects , leaf fleas , Motte scale insects as well as various planthoppers ) collect.
biosynthesis
The biosynthesis of sucrose takes place in the cytoplasm of plant cells of the hexose - intermediates UDP-glucose and fructose 6-phosphate . The two monosaccharides are formed from triose phosphates , which arise as a net gain from the carbon assimilation of photosynthesis ( Calvin cycle ) in the chloroplast . The two triose phosphates glyceraldehyde-3-phosphate and dihydroxyacetone phosphate are either used in the chloroplast for the synthesis of starch (storage starch ) or exported from the chloroplast into the cytosol , where hexoses are formed, which are used to synthesize sucrose (or other carbohydrates or amino acids).
For this purpose, fructose-1,6-bisphosphate is first formed by a condensation reaction between glyceraldehyde-3-phosphate and dihydroxyacetone phosphate , which is then converted to fructose-6-P by dephosphorylation . Glucose-6-P is also formed from fructose-6-P by isomerization , which is activated by subsequent reaction (after previous re-isomerization to glucose-1-phosphate ) with uridine triphosphate (UTP) to uridine diphosphate-glucose ( UDP-glucose ).
The subsequent condensation of UDP-glucose and fructose-6-P to sucrose-6-phosphate is catalyzed by the enzyme sucrose-phosphate synthase . The energy required for this is provided by the cleavage of uridine diphosphate (UDP). Finally, the phosphate residue is split off in an irreversible reaction by the enzyme sucrose phosphate phosphatase, so that sucrose is formed.
Importance as a transport sugar
Sucrose is the most important transport sugar in plants. It is better suited to this than free hexoses, as it is chemically inert as a non-reducing disaccharide . The sucrose produced by photosynthesis in green plant cells when exposed to light reaches the apoplasts through passive transport and then through active transport into the assimilate- conducting phloem of the plant's conducting tissue . In the phloem it becomes other, non-photosynthetic tissues, such as B. growth zones or storage tissues transported.
Other transport sugars are raffinoses in some plant families (e.g. cucurbits , walnuts ) .
Dismantling and recovery
There are different possibilities for the breakdown of sucrose in the target tissues.
In growth zones such as shoot and root tips ( meristems ), sucrose from the phloem is transported symplasmically by plasmodesmata . In the cells, in reverse of the synthesis reaction, the enzyme sucrose synthase cleaves it with UDP to form UDP-glucose and fructose. The two hexoses can be converted to glucose-6-P and z. B. be introduced into glycolysis to generate energy .
In storage tissues, sucrose is transported apoplastically from the phloem to the target cells. It can be taken up by active transport into the cell and broken down there by the sucrose synthase. The majority, however, is split into glucose and fructose by invertases in the cell wall . The two monosaccharides can be taken up by the cell by symporters , where they are transported as glucose-6-P in the chloroplast and used for the synthesis of storage strength.
properties
Chemical properties
The sucrose heard like other sugars to the carbohydrates . It is a disaccharide (double sugar). Sucrose is formed by splitting off water from one molecule each of α- D - glucose ( pyranose form ) and β- D - fructose ( furanose form ). These two molecules are linked to one another via an α, β-1,2- glycosidic bond (glucose α1-2 fructose).
Sucrose is a non- reducing disaccharide. Non-reducing disaccharides are linked to one another via their two anomeric C atoms O -glycosidically, their chemical name ends with -sid . This means that the two components are present in the sucrose molecule in such a way that no aldehyde group can be formed with ring opening (neither from the glucose nor from the fructose molecule). These non-reducing groups of atoms are called acetals . In contrast to hemiacetals, acetals are comparatively stable in a basic and neutral environment. They can only be opened by acid catalysis, the disaccharide z. Sometimes it is split into monosaccharides , resulting in invert sugar (equal parts of glucose and fructose ). Sucrose shows almost no mutarotation due to the lack of ring opening in a neutral environment .
Sucrose therefore shows a negative detection reaction in the Fehling's sample .
Physical Properties
Heating and burning
When sucrose is heated to 185 ° C, it melts and, with decomposition, forms a brownish melt ( caramel ).
Water solubility
Sucrose is very soluble in water. As with most solids, the solubility is temperature-dependent:
| Temperature in ° C | ω sucrose /% | g sucrose / kg water | Density in g / cm³ |
|---|---|---|---|
| 0 | 64.18 | 1792 | 1.31490 |
| 5 | 64.87 | 1847 | 1.31920 |
| 10 | 65.58 | 1905 | 1.32353 |
| 15th | 66.30 | 1970 | 1.32804 |
| 20th | 67.09 | 2039 | 1.33272 |
| 25th | 67.89 | 2114 | 1.33768 |
| 30th | 68.70 | 2195 | 1.34273 |
| 35 | 69.55 | 2284 | 1.34805 |
| 40 | 70.42 | 2381 | 1.35353 |
| 45 | 71.32 | 2487 | 1.35923 |
| 50 | 72.25 | 2604 | 1.36515 |
| 55 | 73.20 | 2731 | 1.37124 |
| 60 | 74.18 | 2873 | 1.37755 |
| 65 | 75.18 | 3029 | 1.38404 |
| 70 | 76.22 | 3205 | 1.39083 |
| 75 | 77.27 | 3399 | 1,39772 |
| 80 | 78.36 | 3621 | 1.40493 |
| 85 | 79.46 | 3868 | 1.41225 |
| 90 | 80.61 | 4157 | 1.41996 |
| 95 | 81.77 | 4486 | 1.42778 |
| 100 | 82.87 | 4872 | 1.43594 |
At 20 ° C a solution with 67% mass fraction ( ω ) (density 1.33 kg / l) is obtained, at 100 ° C, on the other hand, a 83% by weight saturated solution with 83% mass fraction ( ω ) (density 1, 44 kg / l), which no longer separates crystals when it cools down ("hindered crystallization"). It should also be noted that a solution with 60% mass fraction ( ω ) boils at 105 ° C, a solution with 80% mass fraction ( ω ) at 113 ° C and a solution with 90% mass fraction ( ω ) at 132 ° C. (The latter values are taken from the phase diagram of sucrose and water at 100 kPa).
Rotation of polarized light
Sucrose is chiral and therefore optically active : In an aqueous solution, sucrose turns polarized light clockwise ( specific angle of rotation α = + 66.5 ° · ml · dm −1 · g −1 ). The breakdown of sucrose creates a mixture ( invert sugar ) that consists of half glucose and half fructose . This mixture rotates polarized light counterclockwise (specific angle of rotation α = −20 ° · ml · dm −1 · g −1 ), so one observes a reversal of the direction of rotation ("inversion"); the 1: 1 mixture of fructose and glucose is therefore also known as invert sugar .
Analytics
The reliable qualitative and quantitative determination of sucrose is possible after appropriate sample preparation in urine and blood plasma by coupling high-performance liquid chromatography with mass spectrometry . The coupling of gas chromatography with mass spectrometry can also be used for determination in plant material . The sugars to be determined are converted into volatile trimethylsilyl derivatives .
Sweetness
The sweetness is a dimensionless quantity which indicates the relative sweetness of a substance. The sweetness values refer to sucrose, which is assigned a sweetness of 1. The sweetening power is used for a semi-quantitative comparison, particularly with other natural or artificial sweeteners. Sweeteners can have a sweetening power several hundred or thousand times that of sucrose. Interestingly, a derivative of sucrose, D - (+) - sucrose octaacetate , is one of the most bitter compounds known.
Use of sugar as a food
Sucrose is traditionally used in a variety of forms as a food and food additive.
| food | Total carbohydrates including fiber |
Total sugar | Fructose | glucose | Sucrose | Fructose / glucose ratio |
Sucrose in% of total sugar |
|---|---|---|---|---|---|---|---|
| fruit | |||||||
| Apple | 13.8 | 10.4 | 5.9 | 2.4 | 2.1 | 2.0 | 19.9 |
| apricot | 11.1 | 9.2 | 0.9 | 2.4 | 5.9 | 0.7 | 63.5 |
| banana | 22.8 | 12.2 | 4.9 | 5.0 | 2.4 | 1.0 | 20.0 |
| Fig , dried | 63.9 | 47.9 | 22.9 | 24.8 | 0.9 | 0.93 | 0.15 |
| Grapes | 18.1 | 15.5 | 8.1 | 7.2 | 0.2 | 1.1 | 1 |
| orange | 12.5 | 8.5 | 2.25 | 2.0 | 4.3 | 1.1 | 50.4 |
| peach | 9.5 | 8.4 | 1.5 | 2.0 | 4.8 | 0.9 | 56.7 |
| pear | 15.5 | 9.8 | 6.2 | 2.8 | 0.8 | 2.1 | 8.0 |
| pineapple | 13.1 | 9.9 | 2.1 | 1.7 | 6.0 | 1.1 | 60.8 |
| plum | 11.4 | 9.9 | 3.1 | 5.1 | 1.6 | 0.66 | 16.2 |
| vegetables | |||||||
| Beetroot | 9.6 | 6.8 | 0.1 | 0.1 | 6.5 | 1.0 | 96.2 |
| carrot | 9.6 | 4.7 | 0.6 | 0.6 | 3.6 | 1.0 | 77 |
| paprika | 6.0 | 4.2 | 2.3 | 1.9 | 0.0 | 1.2 | 0.0 |
| onion | 7.6 | 5.0 | 2.0 | 2.3 | 0.7 | 0.9 | 14.3 |
| sweet potato | 20.1 | 4.2 | 0.7 | 1.0 | 2.5 | 0.9 | 60.3 |
| yam | 27.9 | 0.5 | traces | traces | traces | - | traces |
| Sugar cane | 13-18 | 0.2-1.0 | 0.2-1.0 | 11-16 | 1.0 | high | |
| sugar beet | 17-18 | 0.1-0.5 | 0.1-0.5 | 16-17 | 1.0 | high | |
| Grain | |||||||
| Corn | 19.0 | 6.2 | 1.9 | 3.4 | 0.9 | 0.61 | 15.0 |
Effect of sugar consumption on the organism
Until the industrial revolution in the 19th century, pure sugar was hardly accessible to broad sections of the population in Central Europe. Sugar was mainly supplied to the body when eating vegetables and fruits as well as honey. It was only since the cultivation of sugar beet around 1800 and the beginning of the industrial refining of sucrose that the organism was confronted with larger amounts of sugar.
High sugar consumption can, especially if it is "free" sugars (English: free sugars ) is - are meant mono- and di-saccharides, which are the foods by the manufacturer, cook or consumer added and naturally in honey, syrups and fruit juices contained sugar - lead to obesity and thus to an increased risk of disease for diabetes mellitus .
Studies by John Yudkin suggest that there is a link between sugar intake and the frequency of heart attacks. It is debated whether sugar promotes the development of cancer and whether a sugar-free diet can hinder the growth of cancer. This thesis has some supporters even among doctors, is actively researched, and there are initiatives for a cancer diet based on a sugar-free or low-sugar diet.
Missing or insufficient dental care after consuming sugary foods leads to the formation of dental caries . Many types of sugar can be converted to tooth-damaging acids by bacteria in the mouth. In particular, sucrose is processed by the bacterium Streptococcus mutans into dextrans , with the help of which these can adhere particularly stubbornly to teeth.
The World Health Organization recommends that so-called free sugars should make up no more than 10% of the daily human energy intake, and ideally should be reduced to 5%. This is mostly exceeded in industrialized countries.
Web links
Individual evidence
- ↑ Entry on SUCROSE in the CosIng database of the EU Commission, accessed on May 22, 2020.
- ↑ a b c d e f Entry on sucrose in the GESTIS substance database of the IFA , accessed on August 21, 2015(JavaScript required) .
- ↑ a b Entry on sucrose. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ^ Brockhaus ABC chemistry. VEB FA Brockhaus Verlag, Leipzig 1965, p. 1221.
- ↑ Albert Gossauer: Structure and reactivity of biomolecules . Verlag Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 340.
- ↑ Helmut Horn, Cord Lüllmann: The great honey book. 3. Edition. Kosmos, Stuttgart 2006, ISBN 3-440-10838-4 , pp. 29-30.
- ^ CA Browne: Handbook of Sugar Analysis. John Wiley and Sons, New York 1912.
- ^ Brockhaus ABC chemistry. Harry Deutsch publishing house, Frankfurt / Zurich 1965.
- ↑ Adalbert Wollrab: Organic Chemistry: An Introduction for Teaching and Minor Students . Springer, 2014, ISBN 978-3-642-45144-7 , pp. 845 .
- ↑ MK Miah, U. Bickel, R. Mehvar: Development and validation of a sensitive UPLC-MS / MS method for the quantitation of [(13) C] sucrose in rat plasma, blood, and brain: Its application to the measurement of blood-brain barrier permeability. In: J Chromatogr B Analyt Technol Biomed Life Sci. 1015-1016, March 15, 2016, pp. 105-110. PMID 26919445
- ↑ P. Kubica, A. Kot-Wasik, A. Wasik, J. Namieśnik, P. Landowski: Modern approach for determination of lactulose, mannitol and sucrose in human urine using HPLC-MS / MS for the studies of intestinal and upper digestive tract permeability. In: J Chromatogr B Analyt Technol Biomed Life Sci. 907, Oct 15, 2012, pp. 34-40. PMID 22985725
- ^ S. Moldoveanu, W. Scott, J. Zhu: Analysis of small carbohydrates in several bioactive botanicals by gas chromatography with mass spectrometry and liquid chromatography with tandem mass spectrometry. In: J Sep Sci. 38 (21), Nov 2015, pp. 3677-3686. PMID 26315495
- ↑ Entry on sweeteners. In: Römpp Online . Georg Thieme Verlag, accessed December 8, 2012.
- ^ Search the USDA National Nutrient Database for Standard Reference. Nal, usda, gov, accessed December 10, 2014 .
- ↑ Ethan B. Butler, Yuhua Zhao, Cristina Muñoz-Pinedo, Jianrong Lu, Ming Tan: Stalling the engine of resistance: Targeting cancer metabolism to overcome therapeutic resistance. In: Cancer Research . Vol. 73, No. 9, 2013, pp. 2709-2717, doi: 10.1158 / 0008-5472.CAN-12-3009 . Retrieved March 13, 2014.
- ↑ Linda C. Nebeling, Edith Lerner: Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. In: Journal of the American Dietetic Association. Vol. 95, No. 6, 1995, pp. 693-697, doi: 10.1016 / S0002-8223 (95) 00189-1 . Retrieved March 13, 2014.
- ↑ U. Schroeder, B. Himpe, R. Pries, R. Vonthein, S. Nitsch, B. Wollenberg: Decline of Lactate in Tumor Tissue After Ketogenic Diet: In vivo microdialysis study in patients with head and neck cancer. In: Nutrition and Cancer . Vol. 65, No. 6, 2013, pp. 843-849, doi: 10.1080 / 01635581.2013.804579 . Retrieved March 13, 2014.
- ↑ Ashraf Virmani, Luigi Pinto, Zbigniew Binienda, Syed Ali: Food, nutrigenomics, and neurodegeneration-neuroprotection by what you eat! In: Molecular Neurobiology. Vol. 48, No. 2, 2013, pp. 353-362, doi: 10.1007 / s12035-013-8498-3 . Retrieved March 13, 2014.
- ↑ Lisa Schönhaar: Those who eat less sugar deprive cancer cells of their nourishment - study shows: You can already make a small change in your diet now to avoid cancer later. In: Business Insider . October 16, 2017, accessed May 31, 2019 .
- ↑ Diet, Nutrition and the Prevention of Chronic Diseases (= WHO Technical Report Series. 916) table 6 on p. 56.
- ↑ WHO guideline: sugar consumption recommendation. In: World Health Organization . March 4, 2015, accessed May 31, 2019 .


