Pseudo asymmetry
Pseudo-asymmetry occurs in compounds that have two different substituents and two enantiomorphic, chiral, but mirror-image substituents at the pseudo-asymmetry center. The term has been in use since 1904. Today the term pseudochirality is usually used. However, there are two optically inactive stereoisomeric mesoforms .
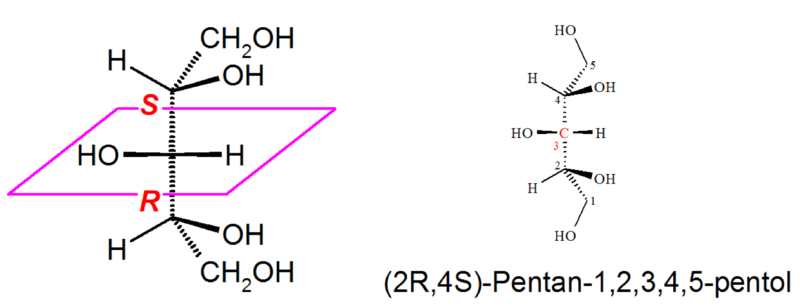
An example is the pentitol xylitol :
The carbon atom C-3, which is central and red in the given molecule, has four different substituents . According to the rules of Cahn, Ingold and Prelog , the following order of priority results:
- first priority: hydroxy ,
- second priority: ( R ) -1,2-dihydroxyethyl- ,
- third priority: ( S ) -1,2-dihydroxyethyl- ,
- fourth priority: hydrogen atom .
It is stipulated that the ( R ) configuration of a substituent (here: 1,2-dihydroxyethyl) has a higher priority than the ( S ) configuration of the same substituent. The molecule shown is therefore r -configured at C-3 . The molecule is still not chiral, it is pseudochiral. Pseudochiral molecules are classified according to Cahn, Ingold and Prelog using the descriptors r - and s -. Pseudochirality centers do not increase the number of enantiomers, but rather the number of mesoforms (in the case of the pentites xylitol and adonitol ).
See also
literature
- Entry on pseudo-asymmetric carbon atom . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04921 .
Individual evidence
- ^ Alfred Werner : Textbook of Stereochemistry 1904 .
- ^ Uwe Meierhenrich : Amino Acids and the Asymmetry of Life , Springer-Verlag, Heidelberg, Berlin 2008. ISBN 978-3-540-76885-2 .
- ↑ Hans Beyer and Wolfgang Walter : Textbook of organic chemistry . 19th edition. S. Hirzel-Verlag, Stuttgart York 1981, ISBN 3-7776-0356-2 .