Descriptor (chemistry)
In chemical nomenclature, a descriptor is a prefix in front of the systematic substance name that describes the configuration or the stereochemistry of the molecule. Some descriptors are only of chemical historical importance and should no longer be used in current publications, as they are no longer taken into account in the current IUPAC recommendations .
Descriptors are often used in combination with locants to uniquely name a chemical structure.
When sorting alphabetically, the descriptors that are usually at the beginning of the systematic name are not taken into account.
Configuration descriptors
cis -, trans -
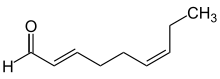
The descriptors cis - (lat. This side ) and trans - (lat. Over ... over (from) , beyond ) are used in the description of chemical configurations in different contexts.
In organic structural chemistry, cis - and trans - can be used to describe the configuration of a double bond , provided that it has a simple substitution pattern with only two radicals. The position of two radicals relative to one another, which are located at different points in a ring system or a larger molecule, can also be described as cis - and trans -, provided that the structure is rigid in terms of configuration and does not allow simple inversion.
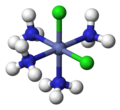
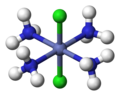
In inorganic complex chemistry, the descriptors cis - and trans - are used to identify the positional isomers in octahedral complexes with A 2 B 4 X configuration or square-planar complexes with A 2 B 2 X configuration.
Square-planar complex: cisplatin
Typographically, cis - and trans - are always written in lowercase and italics .
The cis / trans nomenclature is ambiguous on more highly substituted double bonds and has now largely been replaced by the ( E ) / ( Z ) nomenclature.
With s-cis and s-trans configuration may be a single bond ( s stands for "single bond", single bond) between two double bonds (for example of dienes are described). If both double bonds are on the same side of the single bond, it is an s-cis configuration, if opposing double bonds are in an s-trans configuration. The terms synperiplanar and antiperiplanar are also used for this. However, these are not identical to s-cis or s-trans , but depend on the substitution pattern at the double bonds. In practice, the single bond isomers can hardly be separated due to the low activation energy for the conversion (3.9 kcal / mol for 1,3-butadiene ) and therefore only play a role in reactions that require a certain configuration to proceed, such as the Diels -Alder reaction .
( E ) -, ( Z ) -
The descriptors ( E ) - (from opposite ) and ( Z ) - (from together ) are used to enable a clear description of the substitution pattern on alkenes , cumulenes or other double bond systems such as oximes . For the ascription of ( E ) - or ( Z ) - it is decisive in which positional relationship the two substituents with the highest priority according to the CIP nomenclature are on each side of the double bond system.
The ( E ) - / ( Z ) nomenclature can be applied to any double bond systems (including those with heteroatoms ), but not to substituted ring systems.
The descriptors ( E ) - and ( Z ) - are always written in upper case and in italics, they are surrounded by round brackets, which are set normally as well as additional locants or commas.
o -, m -, p -
 |
 |

|
| o - cresol | m - cresol | p - cresol |
The abbreviations o - (short for ortho , from Greek orthós for upright , straight ), m - ( meta , Greek roughly for between ) and p - ( para , from Greek pará for next to , to the side ) describe the three possible Positional isomers of two substituents on a benzene ring. As a rule, these are two independent individual substituents, but condensed ring systems are also referred to as ortho - annulation or the substitution pattern is included in the naming of the molecule , as is the case with [2.2] paracyclophane, for example . In the current systematic nomenclature, o -, m - and p - are often replaced by specifying locants ( 1,2-dimethylbenzene instead of o- xylene).
o -, m - and p - or also spelled out ortho , meta and para - are always written in lowercase and italics.
exo -, endo -
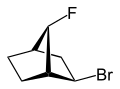
With exo - (from the Greek = outside) or endo - (from the Greek = endon inside) of the relative configuration is bridged bicyclic compounds marked. The position of a substituent in the main ring relative to the shortest bridge (according to IUPAC: the bridge with the highest locant numbers) in the bridged ring system is decisive for the assignment of exo - or endo -. If the substituent to be classified faces this bridge, it is assigned the descriptor exo - if it faces away from the bridge, it is endo - configured.
If there are two different substituents on the same carbon atom, the exo / endo assignment is based on the higher priority according to the CIP rules .
syn -, anti -
If a bridged bicyclic system has a substituent on the shortest bridge, it cannot be assigned an exo or endo descriptor. These isomers are classified using the syn / anti notation. If the substituent to be determined points to the ring with the highest number of ring members (or if it is identical to the ring with the most significant substituent according to the CIP rules), then it is syn -configured (from the Greek syn ... = together ), otherwise the descriptor anti (Greek against ) is assigned to it.
On the other hand, the use of syn - and anti - to indicate the configuration of double bonds, especially in the case of aldoximes and hydrazones derived from aldehydes, is out of date . Here the compounds were referred to as having a syn configuration if the aldehyde-H and the -O (of the oxime) or the -N (of the hydrazone) were in the cis position. These compounds are now described using the ( E ), ( Z ) nomenclature. Aldoximes and hydrazones that used to be classified as syn can therefore now be described as ( E ) -configured.
syn - and anti - are always written in lower case and italic, locants are prefixed and separated with hyphens.
fac -, mer -
The terms fac - (from Latin facies = face) and mer - (from meridonal- ) can be used to specify the arrangement of octahedral complexes with three similar ligands around the central atom. Today this nomenclature is considered out of date, but is still permitted. The prefix fac - describes the situation when the three ligands of the same type occupy the three corners of an octahedron triangular face, whereas in mer -configured complexes the three ligands span a plane in which the central atom also comes to lie.
fac - and mer - are placed in front of the complex name in lowercase and italic.
n -, iso -, neo -, cyclo -
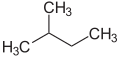
The prefixes n - ( normal -), iso - (from Greek ísos = equal ), neo - (Greek néos = young , new ) and cyclo - (Greek kyklos = circle ) are primarily used to indicate the arrangement of Atoms, mostly of carbon atoms in carbon skeletons, to be described in more detail. n -, iso - and neo - are no longer used in the systematic nomenclature, but are still frequently used in common names and in laboratory jargon .
The prefix n - describes a straight-chain carbon structure without branches, iso - in contrast, a branched structure without specifying this. More generally, iso - stands for a compound that is isomeric to the n -compound, i.e. in which individual atoms or groups of atoms have changed their arrangement.
neo - is a prefix unspecific name for “new”, ie mostly synthetically produced substances or isomers of long-known n -compounds or natural substances (for example neomenthol derived from menthol or neoabietic acid from abietic acid ). According to IUPAC, neo - is only recommended for neopentane or the neopentyl residue.
cyclo - is a frequently used prefix for all cyclic and heterocyclic compounds . In many proper names of chemical substances, cyclo - is not put in front as a prefix, but integrated directly into the name, for example in the case of cyclohexane or cyclooctatetraene .
n -, iso -, neo - are written in lowercase and italics, cyclo - on the other hand, usually only in inorganic compounds. In organic compounds, “Cyclo” is often used as part of the name, is not separated by a hyphen and is also taken into account in alphabetical sorting.
sec -, tert -
The prefixes sec - and tert - are used to identify the substituent environment in a molecule. The exact position of the substituent is not specified, but only the substitution pattern of the neighboring atom (usually a carbon atom). In n -butanol, for example, the OH group is on a primary carbon atom, in sec -butanol on a secondary and in tert -butanol the linking carbon atom is tertiary .
The terms sec - and tert - are considered out of date and are only recommended for unsubstituted sec -Butoxy- and sec -Butyl- or for tert -Butyl-. There are a number of spellings such as "sec.-Butyl-", "s-Butyl-", "sBu-", "Bu s -", which are also considered obsolete.
spiro
In the nomenclature of organic compounds, the prefix “spiro” followed by a Von Baeyer descriptor describes the connection of ring systems with only one common atom, the spiro atom. If the molecule contains several spiroatoms, the prefix “spiro” is given a prefix corresponding to the number of spiroatoms (“dispiro”, “trispiro” etc.). Typographically, "spiro" is set normally.
catena -
The term catena - is used in inorganic nomenclature to describe linear, chain-like polymers made up of identical polyatomic units. An example of this are the catenatriphosphazenes . Related compounds in organic chemistry are the catenanes .
Stereochemical descriptors describing absolute configurations
( R ) -, ( S ) -
The stereochemical descriptors ( R ) - (from Latin rectus = right) and ( S ) - (from Latin sinister = left) serve to clearly describe the absolute configuration of a stereocenter, usually a chiral carbon atom. For this purpose, all substituents at the stereocenter are prioritized according to the CIP rules and the substituent with the lowest priority (“D”) is directed away from the line of sight. If the remaining substituents in descending order of priority ("A" → "B" → "C") describe a circle with direction of rotation to the left, the stereocenter is configured to be ( S ); if the direction of rotation is to the right, the stereocenter is assigned the ( R ) - Configuration assigned.
If a molecule contains several stereocenters, the descriptor must be preceded by a locant (for example in (1 R , 2 S ) -2-amino-1-phenyl-propan-1-ol, the systematic name for norephedrine ). If all stereocenters are configured in the same way, you can dispense with naming the locants in favor of "(all- R ) -" or "(all- S ) -".
Typographically, ( R ) - and ( S ) - are in capital letters and italics; the frequently preceding locants, the surrounding round brackets and commas are set in normal letters.
( r ) -, ( s ) -
The descriptors ( r ) - and ( s ) - are used to describe the absolute configuration of pseudo-asymmetric (pseudochiral) centers. Pseudo-asymmetry occurs when four different substituents are bonded to a carbon atom, two of which differ only in their absolute stereochemical configuration. Examples are the tropane alkaloids and their parent compound tropine , systematically (1 R , 3 r , 5 S ) -8-methyl-8-azabicyclo [3.2.1] octan-3-ol: The carbon atom C-3, on which the - OH group is bound is pseudo-asymmetric; the stereochemical descriptor in the systematic name is written in lowercase and italics.
D -, L -
D - glucose in the Fischer projection.
Red: group with the highest priority,
blue: to determine D - / L - relevant group,
purple: group with achiral carbon atom
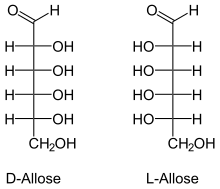
The stereo descriptors D - (from Latin dexter 'right') and L - (Latin laevus 'left') are used to describe the configuration of α- amino acids and sugars . The three-dimensionally structured molecule must first be noted in a clearly defined notation as a two-dimensional image (“ Fischer projection ”). Here, the carbon atom with the highest priority according to the normal nomenclature rules is placed on top and the other carbon chain vertically below. For the assignment of D - or L - that chiral carbon atom is decisive, which is furthest away from the group with the highest priority. If the remainder on this C atom (usually an OH group) is to the left of the carbon chain, the molecule comes from the L series; if the remainder is to the right, it is described with the descriptor D -.
The descriptors D - and L - are written in small caps and separated from the rest of the name with a hyphen.
meso -
The descriptor meso - denotes compounds that have two (or a higher even number) stereogenic centers and can exist in at least one conformation that has a c s symmetry, i.e. H. a rotating mirror axis or mirror plane or point symmetry . Molecules that meet this definition are achiral and optically inactive. An example of this is meso - tartaric acid .
meso - is written in lowercase and in italics in front of the substance name.
( P ) -, ( M ) -
( P ) - Heptahelicene
The enantiomers of axially chiral compounds are identified with the stereochemical descriptors “plus” ( P ) - and “minus” ( M ). The notation ( R ) and ( S ), in more complicated cases also R a and S a , where the index “a” stands for “axial”, is also used.
Helical (helical) molecules (for example the helicenes ) are also described with the descriptors ( P ) - and ( M ) -, whereby molecules with a right-handed helix are assigned the descriptor ( P ) -, and left-handed the descriptor ( M ) -.
The descriptors ( P ) - and ( M ) -, like ( R ) - and ( S ) -, are placed in italics and in round brackets in front of the substance name.
Stereochemical descriptors describing unknown configurations
( RS ) -
If the absolute configuration of a stereocenter is not known, it is described with ( RS ) -, a second stereocenter in the same molecule can then be designated with ( SR ) - to indicate the opposite absolute configuration .
( R * ) -, ( S * ) -
If, on the other hand, the absolute configuration of a stereocenter is known, but one or all of the other stereocenters are unknown, the known configuration serves as a reference; the other stereocenters are marked with ( R * ) - or ( S * ) - according to their relative configuration to the reference center .
Relative configuration of racemates
Sometimes the descriptors ( R * ) and ( S * ) are also used to indicate the relative configuration of racemates, e.g. B. So: (1 R * , 2 R * ) - (±) is a 1: 1 mixture (racemate) of the enantiomer with (1 R , 2 R ) configuration and the enantiomer with (1 S , 2 S )-Configuration. In contrast, (1 S * , 2 R * ) - (±) is a 1: 1 mixture (racemate) of the enantiomer with (1 S , 2 R ) configuration and the enantiomer with (1 R , 2 S ) configuration . This nomenclature is common but does not comply with the IUPAC rules.
(ξ) -, (Ξ) -, ξ-, Ξ-
The Greek letter Xi can also be used to denote a stereocenter of unknown configuration. The typography is adapted to that of the descriptor, which would have to be used in the case of a certain stereochemical configuration: A small ξ- without brackets stands for " c / t -", " cis / trans -", " endo / exo -" or " anti / syn - “; ( r / s ) - however, is replaced by (ξ) - with brackets. Uppercase stands Ξ - for " D -" or " L -" and with brackets ( Ξ ) - for "( R ) -" or "( S ) -" or "( E ) -" or "( Z ) -" .
rel -
If the absolute configurations of the stereocenters in a molecule are not known, but their relative configuration to one another has been determined, they are identified with the prefix rel -. For example, ( rel -2 R , 3 S , 4 R ) -hexane-2,3,4-triol is to be understood as meaning that the molecule is either in the (2 R , 3 S , 4 R ) configuration or as ( 2 S , 3 R , 4 S ) isomer.
The descriptor rel - is written in lowercase and in italics directly in front of the first locant of the systematic substance name.
Stereochemical descriptors for describing mixtures of enantiomers
In systematic names, the lack of clear stereochemical descriptors should be interpreted as an indication of the presence of a racemate.
rac -
The designation rac - in front of the name serves to clarify the fact that an equimolar mixture of enantiomers is present which shows no optical activity. The (±) symbol is synonymous with this.
ambo -
The term ambo - (from Latin ambo = both) is used in place of ( RS ) - or rac -use if the two isomers are not exactly in the ratio 1: 1, but only approximately take this mixing ratio or the exact mixing ratio is unknown is. The descriptor ambo - is written in lower case and italicized in front of the name of the molecule or the partial structure concerned (example: L -alanyl- ambo -leucine).
Stereochemical descriptors describing relative configurations
α-, β-
carbohydrates
The descriptors α- and β- are used in the field of carbohydrate chemistry to describe the configuration of the so-called anomeric carbon atom relative to a reference atom . The C atom is (also called "anomeric center") as the anomeric C-atom aldoses or ketoses denotes that the transition from open-chain to the ring mold (for hemiacetal or halbketalischen pyranose form or furanose form ) of prochiral too new stereocenter. According to the rules of nomenclature in the ring, the anomeric center has the highest priority and therefore has the locant number 1. The reference atom is the chiral atom with the highest locant number within the molecule. If the anomeric center and reference atom are in the same configuration ( R / R ) - or ( S / S ) -, it is the β-anomer, in the ( R / S ) configuration the α-anomer is present.
The anomeric descriptor α or β, separated by a hyphen, is placed directly in front of the configuration descriptor D - or L - and the name of the molecule.
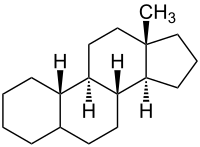
Steroids
The descriptors α - and β - are also used in the context of steroid nomenclature . Here, substituents that are in the half-space above the plane of the ring are usually referred to as β- position, those below the ring plane as α- position. For example, the 13-methyl group in estrane is in the β position.
If the descriptors α - and β - refer to steroids or structurally similar natural substances with linked rings, they are attached directly to the respective locants and are set in italics.
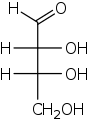
threo -, erythro -
The stereochemical names threo - and erythro - are derived from the configuration of the compounds threose and erythrose and can be transferred to compounds with exactly two neighboring stereocenters. If the residues (mostly OH groups) at these stereocenters are arranged on the same side in the Fischer projection , there is an erythro compound; if the residues are arranged alternately, it is a threo compound.
The IUPAC no longer recommends the use of threo - and erythro -; instead, the more precise ( R ) - / ( S ) or the ( l ) - / ( u ) nomenclature is to be preferred in more complicated cases .
( l ) -, ( u ) -
To classify a compound with two stereocenters as ( l ) - (from like ) or ( u ) - ( unlike ), it is considered whether the configuration of the two stereocenters is identical according to the CIP rules , i.e. ( R , R ) or ( S , S ) is - in this case there is a like ( l ) connection, for ( R , S ) or ( S , R ) a unlike ( u ) connection.
( l ) - or ( u ) - are placed in front of the substance name with small italic letters in round brackets with a hyphen.
ent -, epi -
The prefix ent - (short for enantio -) in front of the name of a chemical compound, usually a natural substance , is used to indicate that the compound described has a stereochemistry that is a mirror image of the stereochemistry of the reference compound (“enantiomer”).
In contrast, the descriptor epi - was used to indicate that exactly one stereocenter is configured as a mirror image of the naturally occurring compound.
allo -
The prefix allo - (from the Greek allos = someone else) is used for several different things. In the area of carbohydrates, it characterizes a relative configuration of four stereogenic centers, which corresponds to that of allose , in which all OH groups are on the same side of the carbon chain in the Fischer projection.
In the area of the nomenclature of amino acids with two chiral centers, the name was assigned in the past to the amino acid diastereomer that was discovered first. The common name of the diastereomer found or synthesized below was then given the prefix allo -. This method was only used with trivial names, not with semi-systematic or systematic names and is now only recommended for allo - isoleucine and allo - threonine .
As a prefix, allo is always placed in front of the name in lowercase and italic, but there are chemical compounds in which “Allo-” is part of the name (for example, Allocinnamic acid or Alloocimen ), here it is to be avoided that “Allo” is prefixed with lowercase and italic becomes.
Descriptors for describing physical properties
(+) -, (-) -, (±) -
A physical property of non-racemic mixtures of chiral compounds is their ability to rotate the plane of polarized light through a certain angle. This angle of rotation depends on the concentration of the solution, the solvent and the length of the measuring path.
In order to name chiral compounds, the sense of rotation of the optical rotation can therefore also be given (in addition to the systematic name and the absolute stereochemical configuration). For this purpose, the substance name is preceded by one of the characters (+) - or (-) -.
Racemic mixtures of compounds with only one stereocenter are described with a prefixed (±) -.
No direct conclusions about the absolute configuration of a molecule can be drawn from the (+) - / (-) - specification, the descriptors (+) - and (-) - are neither with ( R ) - and ( S ) -, nor with D - and L - equivalent. From the descriptor (±) - it follows, however, that a mixture of substances (racemate = 1: 1 mixture of two enantiomers) is present, i.e. H. (±) - is equivalent to the descriptors ( RS ) - and DL -.
Subtraction prefixes
Subtraction prefixes such as Des- , Nor- and Anhydro- indicate that something has been split off from a parent compound or that something is “missing”.
See also
Individual evidence
- ↑ Entry on stereodescriptor . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S05976 Version: 2.3.1.
- ↑ Entry on cis-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on trans-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ IUPAC rules E-2, E-3 (PDF; 542 kB).
- ↑ IUPAC rule R-7.1.1 .
- ↑ Entry on cis, trans . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01092 Version: 2.3.2.
- ↑ Entry on s-cis-. In: Römpp Online . Georg Thieme Verlag, accessed on October 20, 2019.
- ↑ entry to E, Z . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E01882 Version: 2.3.2.
- ↑ Entry on Ortho-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on Met (a) .... In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on Para-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on exo-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on endo-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ a b Entry on endo, exo, syn, anti . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E02094 Version: 2.3.2.
- ↑ a b entry on syn-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on Anti-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on fac-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ^ Entry on Mer. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on fac- . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.F02313 Version: 2.3.2.
- ↑ entry to mer- . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M03828 Version: 2.3.2.
- ↑ Entry on Iso .... In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ^ Entry on Neo .... In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on Cyclo .... In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ IUPAC rules A-2.1, A-2.25 .
- ↑ a b c IUPAC rule R-9.1, Table 19b .
- ↑ Entry on cyclo- . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01495 Version: 2.3.2.
- ↑ IUPAC rules A-2.25 , C-205.1 , R-5.5.1.1 .
- ↑ IUPAC rule R-9.1 , Table 26b.
- ^ IUPAC rule A-2.25 .
- ↑ Entry on sec-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on tert-butyl .... In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ^ IUPAC: Nomenclature of Spiro Compounds , accessed on May 23, 2016.
- ↑ Entry on catena- . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00903 Version: 2.3.2.
- ↑ Entry on catena-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ S. Gorter and GC Verschoor: The crystal structure of catena-tri-µ 2 - (1,12-dodecanedinitrile) copper (II) hexachloroantimonate (V) Cu (C 12 H 20 N 2 ) 3 (SbCl 6 ) 2 . In: Acta Cryst. (1976). B32, 1704-1707, doi : 10.1107 / S0567740876006262 .
- ↑ IUPAC rules D-4.4, I-9.7.3 and I-10.8.3.5.
- ↑ Entry on CIP rules. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ entry relating to R, S . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.R05423 Version: 2.3.2.
- ↑ Entry on pseudo-asymmetric carbon atom . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04921 .
- ↑ Entry on d, l, dl . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.D01512 Version: 2.3.2.
- ^ Entry on Fischer – Rosanoff convention (or Rosanoff convention) . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.F02392 Version: 2.3.2.
- ↑ Entry on d. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ K.-H. Hellwich: stereochemistry. Springer, 2007, ISBN 3540717080 . P. 11 ( limited preview in Google Book search).
- ↑ Bernhard Testa: Fundamentals of Organic Stereochemistry , Verlag Chemie, Weinheim, 1983, pp. 54–56, ISBN 3-527-25935-X .
- ↑ Entry on meso-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on meso . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.M03838 Version: 2.3.2.
- ↑ entry to axial chirality . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00547 Version: 2.3.2.
- ↑ Entry on helicity . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.H02763 Version: 2.3.2.
- ↑ a b entry on RS. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on relative configuration . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.R05260 Version: 2.3.2.
- ↑ Entry on configuration. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Bernhard Testa: Fundamentals of Organic Stereochemistry , Verlag Chemie, Weinheim, 1983, pp. 57–58, ISBN 3-527-25935-X .
- ↑ IUPAC: Nomenclature and Symbolism for Amino Acids and Peptides (Recommendations 1983) (PDF; 539 kB), p. 601.
- ↑ Entry on ξ. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on Ξ. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on rel-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on relative configuration . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.R05254 Version: 2.3.3.
- ↑ Entry on rac-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on racemate . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.R05025 Version: 2.3.2.
- ↑ Entry on ambo-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on ambo . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00261 Version: 2.3.2.
- ↑ Entry on anomers. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ a b Entry on α (alpha), β (beta) . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00003 Version: 2.3.2.
- ^ IUPAC: Nomenclature of Carbohydrates 2-Carb-6 and 2-Carb-7 (Recommendations 1996).
- ^ GP Moss: Nomenclature of steroids (Recommendations 1989) . In: Pure and Applied Chemistry . Volume 61, Issue 10, pp. 1783-1822, doi : 10.1351 / pac198961101783 .
- ↑ Entry on threo-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on Erythr (o) .... In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ^ Entry on erythro, threo . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E02212 Version: 2.3.2.
- ↑ entry to l, u . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.L03423 Version: 2.3.2.
- ↑ entry to un-. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ^ IUPAC rules F-6.4, RF-10.3
- ↑ Entry on epi-. In: Römpp Online . Georg Thieme Verlag, accessed on May 6, 2013.
- ↑ a b Entry on Allo…. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.
- ↑ Entry on allo-in amino-acid nomenclature . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00239 Version: 2.3.2.
- ↑ Entry on optical activity. In: Römpp Online . Georg Thieme Verlag, accessed on September 14, 2012.