Half acetals
| Half acetals |
|---|

|
| General structure of the hemiacetals with the blue marked alkoxy / aryloxy and hydroxyl groups , both of which are bonded to the same carbon atom. The radical R 1 represents an aliphatic , cyclic or aromatic radical or a hydrogen atom . The radical R 2 is not a hydrogen atom, since otherwise it is a geminal diol. |
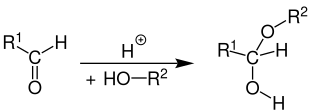
Hemiacetals (or hemiacetals ) are organic compounds , which are characterized by an alkoxy group or aryloxy group -OR, and a hydroxy group are distinguished -OH, that are attached to the same carbon atom. Hemiacetals are formed as an intermediate stage in acetal formation by adding an alcohol to a carbonyl group with acid or base catalysis. Under the action of strong acids, an acetal is finally formed through reaction with another molecule of the alcohol . They have the general structure R 1 R 2 C (OH) OR, where R not H can be. Cyclic hemiacetals are called lactols .

Natural substances
Numerous sugars are aldoses , such as D - glucose . The aldoses are often in the form of cyclic hemiacetals.
Manufacturing
Overview reaction
Aldehydes (and ketones in the case of hemiketal formation) react under acid or base catalysis with alcohols in a nucleophilic addition reaction with one another and initially form hemiacetals as an intermediate stage in acetal formation ( ketal formation ).
Reaction mechanism
The following reaction scheme is intended to clarify the mechanistic details of the acid-catalyzed hemiacetal formation:
In the first step, the carbonyl group of the aldehyde is protonated on the nucleophilic oxygen ( 1 ). A mesomeric stabilized intermediate ( 2 ) is formed. An oxonium ion and a carbenium ion are present in parallel here . An alcohol molecule can nucleophilically attach to the latter . An oxonium ion is formed again as an intermediate ( 3 ), which reacts to form the hemiacetal by splitting off the proton ( 4 ). The radical R 1 in the reaction scheme represents either an aliphatic , cyclic or aromatic radical or a hydrogen atom . The radical R 2 is also an aliphatic, cyclic or aromatic radical, but not a hydrogen atom.
Dithio half acetals
If you replace the two oxygen atoms in half-acetals with sulfur atoms, you get dithio half-acetals. A dithio hemiacetal played a central role in the synthesis of the natural product erythromycin . Dithio half acetals characterize the odor of the tropical fruit durian .
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 358, ISBN 3-342-00280-8 .
- ↑ Entry on hemiacetals . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.H02774 Version: 2.1.5.
- ↑ K. Peter C. Vollhardt, Neil E. Schore, Katrin-M. Roy, Holger Butenschön: Organic Chemistry . 5th edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2011, p. 850, ISBN 978-3-527-32754-6
- ↑ RB Woodward , E. Logusch, KP Nambiar, K. Sakan, DE Ward, BW Auyeung, P. Balaram, LJ Browne, PJ Card, CH Chen, RB Chenevert, A. Fliri; K. Frobel, HJ Gais, DG Garratt, K. Hayakawa, W. Heggie, DP Hesson, D. Hoppe , I. Hoppe, JA Hyatt, D. Ikeda, PA Jacobi, KS Kim, Y. Kobuke, K. Kojima, K. Krowicki, VJ Lee, T. Leutert, S. Malchenko, J. Martens , RS Matthews, BS Ong, JB Press, TV Rajanbabu, G. Rousseau, HM Sauter, M. Suzuki , K. Tatsuta, LM Tolbert, EA Trusdale, I. Uchida, Y. Ueda, T. Uyehara, AT Vasella, WC Vladuchick, PA Wade, RM Williams, HNC Wong: Asymmetric Total Synthesis of Erythromycin. 1. Synthesis of an Erythronolide A Seco Acid Derivative via Asymmetric Induction , J. Am. Chem. Soc. 1981 , 103 , 3210-3213, doi : 10.1021 / ja00401a049 .
- ↑ Volker Mrasek : The secret of the stink fruit - German researchers investigate the Asian durian , Deutschlandfunk " Forschung aktuell " from February 11, 2013.