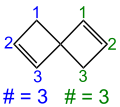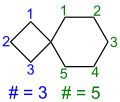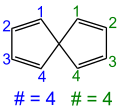Locant

In chemical nomenclature, locants are used to precisely describe certain positions of atoms or bonds within a molecule. They are used, for example, to identify the branch points in a carbon chain within a compound or to designate the positions of heteroatoms , double bonds or triple bonds . The set of locants in a connection is called its locant set .
Different locant types are used for different areas of application.
Arabic numbers
Aliphatic compounds
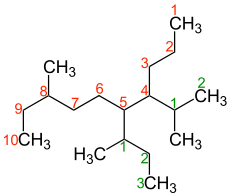
The most common type of locant used in chemical nomenclature are Arabic numbers. For example, each carbon atom in an organic molecule is assigned a number, with the longest carbon chain in the case of saturated aliphatic hydrocarbons indicating the parent system; all shorter branches are considered side chains . The master chain is numbered so that the locants of the branch points are as small as possible. The side chains are also numbered consecutively, whereby the linking atom with the parent chain always receives the number 1. In alkyl radicals, the “yl” position (carbon atom with the free valence) is given the locant 1 when numbering the chain.
Cyclic compounds
Chemical compounds that also contain rings in the atomic structure ( cyclic compounds ) are also provided with Arabic numerals as locants. If there are multinuclear ring systems ( polycycles ) or if heteroatoms are contained in the cyclic parts of the molecule ( heterocycles ), trivial names are often widespread, for which precise rules for locant positioning are specified. Clear compilations often help to assign the valid position numbering of single-core and simple multi-core ring systems.
Italicized Latin capital letters
Italicized Latin capital letters are used to mark the position of heteroatoms in the molecule . In fact, these are the element symbols in italics , so a combination of uppercase and lowercase letters is possible.
For example, the compound N , 2-dimethylaniline has two methyl radicals on the aniline body , whose positions on the nitrogen atom are clearly assigned to the amino function (denoted by the italic “N”) and to the C-2 carbon atom of the aromatic (denoted by the Arabic number 2) become. If there are two (or more) heteroatoms of the same type in the molecule, they are differentiated with dashes, as is the case with N , N ′ -dimethylurea, for example . If a clear assignment cannot be achieved with this, the heteroatom symbols in italics are supplemented by superscript locant digits , as in N 4 , N 4 -dimethylcytidine ; this shows which nitrogen atom the two methyl groups are bound to.
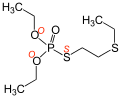
In the case of thiophosphoric acid esters, the element symbols of the heteroatoms in italics in front of the systematic names are used to assign the atom via which the ester groups are bound. This is clear from the regioisomers demetone-O ( O , O- diethyl- O- (2-ethylthioethyl) thiophosphate) and demetone-S ( O , O -diethyl- S - (2-ethylthioethyl) thiophosphate). In the following gallery the locants in the structural formulas and the systematic names are marked in red :
Demeton-O [systematic: O , O -diethyl- O - (2-ethylthioethyl) thiophosphate]
Demeton-S [systematic: O , O -diethyl- S - (2-ethylthioethyl) thiophosphate]
4 H -1,2,4- triazole
Normally set Latin capital letters

Compounds whose structure is derived from the carbon backbone of the sterane are called steroids and are described using the steroid nomenclature . The rings of Steran scaffold, which systematically as cyclopentylsubstituiertes perhydro phenanthrene may be considered are, besides, as with normal set provided Roman alphabet that the three linked six-membered rings "A", "B" and "C" and the subsequent five-membered ring with "D" are designated.
Greek lowercase letters
In the past, relative distances between functional groups were occasionally indicated by Greek lowercase letters. Today, this nomenclature variant is considered to be semi-systematic, but is still widespread , especially in the area of unsaturated fatty acids . The atom directly adjacent to the functional group is assigned an “α”, the following “β” etc. up to the most distant one, which is always described as “ω” regardless of the absolute distance.
The acronym " amphetamines " is an example of this type of locants: The semi-systematic name of this group of compounds is α - M ethyl ph enyl et hyl amine ; the "α" thus indicates the direct proximity to the amino group in the molecule. In γ-aminobutyric acid , the position of the amino group relative to the carboxy group is emphasized. The ring sizes of cyclic amides ( lactams ) and cyclic esters ( lactones ) are named similarly .
The position of double bonds relative to an adjacent functional group is also often indicated by means of lower-case Greek letters. For example, 2-cyclohexenone is also referred to as an α, β-unsaturated ketone.
Locants in square brackets
Spiro compounds
Spiro compounds are characterized by at least two or more rings that are only connected by a common bridgehead atom, the so-called spiro atom . In the case of monospiro compounds (only one spiro atom), the ring sizes of the rings involved are put in square brackets in the formation of the systematic name after the term spiro , whereby the spiro atom is not counted in each case . The two resulting numbers are separated by a period. The expression in brackets is also referred to as the Von-Baeyer descriptor according to the Von-Baeyer nomenclature .
If the molecule contains several spiroatoms, the prefix “spiro” is given a prefix corresponding to the number of spiroatoms ( dispiro , trispiro etc.). The Von Baeyer descriptor is formed by first starting with only one spiroatom on one of the rings and specifying the number of its ring members. In the following, the molecule is circled and the number of atoms between the spiro atoms is indicated, separated by dots, until the initial ring is reached again. Whenever a spiroatom is touched for the second time, the connection of the ring member just mentioned is indicated by the superscript locant number of the spiroatom to which the bridge is attached. In the case of asymmetrical molecules, the name is formed in such a way that the smallest possible locant digits are selected for the spiro atoms.
Bicyclic and bridged compounds
For the systematic naming of bicyclic systems, the number of ring members (without bridgehead atoms ) is placed in square brackets in descending order after the term bicyclo . For example, 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) is a bicyclic compound with two nitrogen heteroatoms, which is composed of a 7, a 6 and a 2 ring (ie without bridgehead atoms 0 , i.e. direct connection).
In the case of additionally bridged systems , the number of ring members reduced by 2 in square brackets applies analogously; however, locants must be mentioned here from the fourth ring that clearly identify the connection points. They are superscripted and appended, separated by commas. The systematic name for dicyclopentadiene is tricyclo [5.2.1.0 2,6 ] deca-3,8-diene, in addition to the bicyclic system there is a 7-, a 4- and a 3-ring (ring size without bridgehead atoms: 5, 2.1) a direct link (ring size 0) between atoms 2 and 6. The systematic name for quadricyclane , tetracyclo [2.2.1.0 2.6 .0 3.5 ] heptane, is formed analogously .
Linked polycyclic hydrocarbons
A so-called fusion descriptor is used to identify the linkage position of polynuclear ring systems in polycyclic aromatic hydrocarbons , for example in indeno [1,2,3- cd ] pyrene . This is made up of the locant numbers, which describe the connection point in one part of the molecule, and the bonds designated with italic Latin small letters, which describe the connection point in the other part of the molecule. If there are molecular parts with high symmetry (for example benzene and pyrene ) the fusion descriptor can be greatly simplified, here to benzo [ a ] pyrene .
literature
- IUPAC: Preferred IUPAC Names , Chapter P-1, Nomenclature of Organic Compounds , pp. 26-40.
Individual evidence
- ↑ IUPAC: Rule R-0.2.4.2: Lowest set of locants
- ↑ Philipp Fresenius and Klaus Görlitzer: Organic-chemical nomenclature , Wissenschaftliche Verlagsgesellschaft Stuttgart, 1991, 3rd edition, p. 28, ISBN 3-8047-1167-7 .
- ↑ IUPAC: Rule A-22: Fused Polycyclic Hydrocarbons - Numbering
- ↑ maybridge.com: Numbering of multiple ring system
- ^ GP Moss: Nomenclature of steroids (Recommendations 1989). In: Pure and Applied Chemistry. 61, 1989, pp. 1783-1822, doi : 10.1351 / pac198961101783 .
- ^ IUPAC: Extension and Revision of the Nomenclature for Spiro Compounds , Recommendations 1999.
- ↑ IUPAC rule A-31.2
- ↑ IUPAC: Rule R-2.4.1: Fusion nomenclature