Carbon chain


In organic chemistry, the term carbon chain describes a structural element of compounds in which several carbon-carbon bonds occur, i.e. the carbon atoms are chained to one another . A distinction is made between linear and branched chains. The carbon atoms are given different names depending on their position in the chain: A primary carbon atom has only one additional carbon atom as a neighbor, a secondary carbon atom has two, a tertiary carbon atom has three and a quaternary carbon atom has four.
presentation
There are different ways of representing carbon chains. All atoms bound to the chain are drawn in valence line formulas . Often the hydrogen atoms linked to the carbon chain are omitted and only the CC bonds are shown; this notation is called the skeletal formula .
The carbon atoms can be linked via covalent single bonds , as in alkanes , for example . The molecules can rotate freely around the axis of this bond. If two carbon atoms are linked by a double bond , as in the case of alkenes , the free rotation of this bond is suspended. In alkynes , two carbon atoms are linked by a triple bond . A special case are ring-shaped connections such. B. Cycloalkanes : Here the carbon chain is closed. There are various ring-shaped compounds made up of different numbers of carbon atoms. The rotatability of the bindings is severely restricted in the case of small rings, but increases with the size of the ring.
| propane | Propene | Propyne | Cyclopropane |
|---|---|---|---|
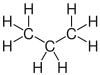
|

|
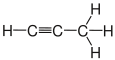
|
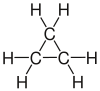
|
Also, polymers may comprise as structural element carbon chains. Polyethylene and polypropylene, for example, consist exclusively of long-chain hydrocarbon molecules. The properties of the polymers depend on the length of the carbon chain, its structure (regularity, branching) and the copolymers or additives used .
Van der Waals forces act between the non-polar carbon chains , i. H. Induced dipoles lead to the formation of weak bonds between the individual molecules, the van der Waals bonds. This can be clearly seen in the case of the alkanes, whose boiling point increases with the chain length.
Significance for the nomenclature of organic compounds
In the systematic naming ( IUPAC nomenclature) of organic-chemical four bonds, the determination of the longest carbon chain (open-chain or ring-shaped) plays an important role. The stem name of such a compound is derived from the name of the longest carbon chain in the molecule, which is then specified more precisely using prefixes , suffixes, affixes, etc.
Other elements that can form chain-like connections:
Web links
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, pp. 24-27, ISBN 3-342-00280-8 .