Fatty acids
Fatty acids are aliphatic monocarboxylic acids with mostly unbranched carbon chains that are either saturated or unsaturated . The term "fatty acids" is based on the knowledge that natural fats and oils consist of the esters of long-chain carboxylic acids with glycerine . From this point of view, fatty acids are also counted among the lipids . Later all other alkyl carboxylic acids and their unsaturated representatives were assigned to the fatty acids.
General and structure
Fatty acids differ in the number of carbon atoms (chain length) and - in the case of unsaturated fatty acids - in the number and position of double bonds . Due to their chain lengths, fatty acids can be broken down into lower , short-chain fatty acids (up to 6-8 carbon atoms) (SCFA; Short Chain Fatty Acids), the lower limit being interpreted differently here, either 1, 2, 3 or 4 carbon atoms, medium , medium-chain (6-8 to 12 C-atoms) (MCFA; Middle Chain Fatty Acids) and higher , long-chain (13 to 21 C-atoms) (LCFA; Long Chain Fatty Acids) fatty acids are divided. Fatty acids with more than 22 carbon atoms are also known as VLCFAs (Very Long Chain Fatty Acids).
The naming as "fatty acid" suggests that an individual compound must once have been a component of a fat in order to be a fatty acid, but this is not necessarily the case. Today, this term covers carboxylic acids with (chain-like) organyl groups.
Natural fatty acids usually consist of an even number of carbon atoms and are unbranched. Exceptions to this, however, can be found in all realms . One definition is that the carbon chain must be at least four carbon atoms long; thereafter butyric acid is the simplest natural fatty acid. Another definition uses the formula CH 3 (CH 2 ) x COOH, where x is the number of carbon atoms in the hydrocarbon chain; only three carbon atoms can be present here if x = 1, or only two if x = 0; however, when x = 0, strictly speaking, one cannot speak of a “chain”. Fatty acids with C = C double bonds are called "unsaturated". This double bond is usually cis -configured . If there are two or more double bonds, they are usually separated from one another by a methylene group (-CH 2 -).
A large variety of fatty acids (more than 400 different structures, of which only about 10–12 are common) occur - mostly in the form of triacylglycerides , i.e. esterified with glycerine - in the seed oils of the vegetable kingdom. Rare fatty acids, which occur esterified in larger percentages in seeds of certain plant families, can illustrate developmental relationships (kinship relationships, chemotaxonomy, evolution; see e.g. also world economy) such as petroselinic acid , tariric acid , erucic acid , cyclopentene fatty acids and cyclopropene fatty acids . Some types of bacteria can be differentiated based on their fatty acid composition.
Essential fatty acids refer to fatty acids that an organism needs but cannot produce itself. For mammals , those fatty acids are essential that have one or more double bonds at positions higher than C-9 (counting from the carbonyl carbon) because they lack the enzymes to insert such double bonds. Strictly speaking, these are only linoleic acid and α-linolenic acid for humans .
In the food industry, fatty acids are mainly used as raw materials for various emulsifiers , but also as carriers , separating agents (e.g. in chewing gum) or as coating agents (e.g. for fruit). They are generally approved in the EU as food additives with the collective name E 570 without maximum quantity restriction (quantum satis) for foodstuffs.
The sodium or potassium salts of the higher fatty acids are known as soaps and are used as surfactants .
Saturated and unsaturated fatty acids

A saturated fatty acid ( SFA , of Engl. Saturated fatty acids ) is - as a group of alkanoic acids - a fatty acid that no double bonds between carbon atoms has. The saturated fatty acids form a homologous series with the empirical formula C n H 2n + 1 COOH.
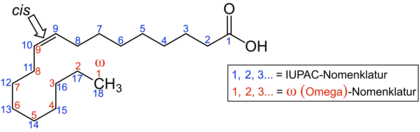
Unsaturated fatty acids have as alkenoic acids , at least one C = C double bond ( MUFA , of Engl. Monounsaturated fatty acids ). Polyunsaturated fatty acids ( PUFA , of Engl. Polyunsaturated fatty acids ) have two or more double bonds between the carbon atoms of the chain. Since the double bonds in natural fatty acids are mostly in the cis configuration , there is a bend of around 30 ° in the hydrocarbon chain. As a result, the van der Waals interaction with other molecules is weakened; the melting point is lowered. Some unsaturated fatty acids are essential for humans because the human body cannot synthesize them but needs them. These include fatty acids that have double bonds in certain positions, the omega- n fatty acids .
A distinction is made between simple (monoic acids), double (dienoic acids), triple (trienoic acids) or poly (polyenoic acids) unsaturated fatty acids.
Nomenclature ( cis , trans , ω)
In the omega-n fatty acids, n stands for a number and describes the position of one of the double bonds. The omega-counting method, which is often used in food chemistry, counts from the “ ω- end” of the carbon chain that is opposite the carboxy group. The double bond near the carboxy group therefore receives the largest number; the position of the double bond closest to the ω end determines the type of omega n fatty acid. In the illustration of linolenic acid, the ω-counting method is shown in red . Only the double bond counted first is decisive for the classification into the various groups of omega n fatty acids.

In addition to unsaturated fatty acids in the cis configuration, in rare cases there are also fatty acids with trans -configured double bonds, the trans fatty acids . Glycerides of trans -fatty acids are sometimes produced as an undesirable by-product in margarine production and are suspected of having harmful properties. In particular, the negative influence on coronary heart disease is cited in the literature .

If there are several double bonds - more precisely C = C double bonds - in a fatty acid, these are usually - analogous to the linolenic acid shown above on the right - separated from one another by a methylene group ( CH 2 group ); these are then referred to as isolenic acids . Are the double bonds by two or more methylene groups separated from each other, it is called these particular fatty acids to - or polymethylene interrupted or non-methylene-interrupted Isolensäuren (NMI; Non-Methylene-Interrupted or PMI; poly-Methylene-Interrupted).
However, there are also conjugated fatty acids (conjugic acids) in which the double bonds are closer to one another, namely conjugated . In the illustration of the rumenic acid octadeca-9c, 11t-dienoic acid, the double bonds are conjugated. Since one of the double bonds is present here as a trans double bond , this is also a trans fatty acid. Bacteria in the digestive tract of ruminants are often responsible for the formation of these fatty acids . Conjugated fatty acids are therefore found in all dairy products.
Odd-numbered fatty acids
Fatty acids with an uneven number of carbon atoms are of little importance and arise, among other things, from α-oxidation from fatty acids with even-numbered carbon atoms. In humans, this mainly affects phytanic acid and pristanic acid , which are then broken down into propionyl-CoA in the β-oxidation .
Branched Fatty Acids

Lower fatty acids with branches in the carbon chain are found in some essential oils . Valerian extracts contain esters of isovaleric acid .
Phytanic acid (3,7,11,15-tetramethylhexadecanoic acid) is a branched-chain carboxylic acid that occurs as a breakdown product of chlorophyll . Traces of this compound can be found in many foods (e.g. milk ). The pathological inability to break down this carboxylic acid leads to Refsum's syndrome .
Branched-chain fatty acids are found in the membranes of numerous prokaryotes . Their occurrence is used to identify a type of bacteria and to investigate related relationships between the organisms. Above all, fatty acids with a methyl group as a branch near the “ω-end” of the carbon chain are important, such as iso- pentadecanoic acid (methyl group on the penultimate carbon atom) and the anteiso- pentadecanoic acid (methyl group on the penultimate carbon atom). They are also found in small amounts in milk fat . It is assumed that they are produced by bacteria in the rumen and are ingested by the cows and stored in their fatty tissue or in milk fat.
Branched chain fatty acids are also known as (BCFA; Branched Chain Fatty Acids).
Cyclic fatty acids
Cyclic fatty acids or CFAM (Cyclic Fatty Acid Monomers) are fatty acids with an intramolecular ring of 3, 5 or 6 C units, saturated or with double bond (s) in the ring. E.g. sterculic acid and chaulmoogric acid; see under other fatty acids.
List of fatty acids and shorter monocarboxylic acids
| Saturated fatty acids and shorter monocarboxylic acids | ||||||
|---|---|---|---|---|---|---|
| Number of carbon atoms: double bonds | Common name | Chemical name | Gross formula | Occurrence | Melting point | boiling point |
| 1-0 | Formic acid | Methanoic acid | HCOOH | Widespread, in almost all organisms | 8.3 ° C | 101 ° C |
| 2-0 | acetic acid | Ethanoic acid | CH 3 COOH | Vinegar (by oxidation of ethanol) | 16.6 ° C | 118 ° C |
| 3-0 | Propionic acid | Propanoic acid | C 2 H 5 COOH | Intermediate product in methane fermentation | −20.5 ° C | 141 ° C |
| 4-0 | Butyric acid | Butanoic acid | C 3 H 7 COOH | Milk fat , sweat, the simplest fatty acids | −5.1 ° C | 164 ° C |
| 5-0 | Valeric acid | Pentanoic acid | C 4 H 9 COOH | Valerian root , wood vinegar | −34 ° C | 186 ° C |
| 6-0 | Caproic acid | Hexanoic acid | C 5 H 11 COOH | Milk fat, arises from butyric acid fermentation | −4 ° C | 205 ° C |
| 7-0 | Enanthic acid | Heptanoic acid | C 6 H 13 COOH | as an ester in calamus oil ( Acorus calamus ) | −7.2 ° C | 222 ° C |
| 8-0 | Caprylic acid | Octanoic acid | C 7 H 15 COOH | Milk fat, coconut fat | 16.5 ° C | 239 ° C |
| 9-0 | Pelargonic acid | Nonanoic acid | C 8 H 17 COOH | essential oil of Pelargonium roseum , cheese, fusel oil , wine | 12.4 ° C | 254 ° C |
| 10-0 | Capric acid | Decanoic acid | C 9 H 19 COOH | Animal and vegetable fats | 31.4 ° C | 269 ° C |
| 11: 0 | - | Undecanoic acid | C 10 H 21 COOH | Essential oils ( Iris and Quendel oil Thymus serpyllum ) | 28.5-29.3 ° C | 280 ° C |
| 12: 0 | Lauric acid | Dodecanoic acid | C 11 H 23 COOH | Milk fat, vegetable fats | 43.8 ° C | 225 ° C (100 torr) |
| 13: 0 | - | Tridecanoic acid | C 12 H 25 COOH | Vegetable oils | 41.5 ° C | 236 ° C (100 torr) |
| 14: 0 | Myristic acid | Tetradecanoic acid | C 13 H 27 COOH | Milk fat, fish oil , animal and vegetable fats | 54.2 ° C | 250 ° C (100 Torr) |
| 15: 0 | - | Pentadecanoic acid | C 14 H 29 COOH | Ruminant milk and body fat, fish oil | 52.3 ° C | 257 ° C (100 torr) |
| 16: 0 | Palmitic acid | Hexadecanoic acid | C 15 H 31 COOH | Animal and vegetable fats | 62.5-63 ° C | 351.5 ° C |
| 17: 0 | Margaric acid | Heptadecanoic acid | C 16 H 33 COOH | Animal and vegetable fats | 61.3 ° C | 227 ° C (100 torr) |
| 18: 0 | Stearic acid | Octadecanoic acid | C 17 H 35 COOH | Animal and vegetable fats | 69.2-69.9 ° C | 350 ° C (dec.) |
| 19: 0 | - | Nonadecanoic acid | C 18 H 37 COOH | Beef fat, dill ( Anethum graveolens ) | 69.4 ° C | 297 ° C (100 torr) |
| 20: 0 | Arachidic acid | Eicosanic / icosanoic acid | C 19 H 39 COOH | in small amounts in plant seeds and animal fats | 75.5 ° C | 328 ° C (dec.) |
| 21: 0 | - | Heneicosanoic acid | C 20 H 41 COOH | in fungi of the genus Armillaria (4–5% of the fatty acids) and in a few plants | 74-75 ° C | |
| 22: 0 | Behenic acid | Docosanoic acid | C 21 H 43 COOH | in small amounts in plant seeds and animal fats, in Gaucher's disease | 79.5-80.5 ° C | |
| 24: 0 | Lignoceric acid | Tetracosanoic acid | C 23 H 47 COOH | Wood, tar , peanut oil , some vegetable fats, part of the sphingomyeline | 81.5-84.5 ° C | |
| 26: 0 | Cerotic acid | Hexacosanoic acid | C 25 H 51 COOH | Beeswax , carnauba wax , montan wax , wool sweat | 87.7-88.5 ° C | |
| 28: 0 | Montanic acid | Octacosanoic acid | C 27 H 55 COOH | Montan wax , beeswax, china wax | 91-93 ° C | |
| 30: 0 | Melissic acid | Triacontanoic acid | C 29 H 59 COOH | Beeswax, Selinum , Trichosanthes and Pericampylus species | 92-94 ° C | |
| 32-0 | Laccic acid | Dotriacontanoic acid | C 31 H 63 COOH | in growing | 96 ° C | |
| 34: 0 | Geddic acid | Tetratriacontanoic acid | C 33 H 67 COOH | in growing | 98.4 ° C | |
| Monounsaturated fatty acids | ||||||
|---|---|---|---|---|---|---|
| Number of carbon atoms: double bonds | Common name | Gross formula | Position of the double bond |
Occurrence | Melting point | Chemical name |
| 11: 1 | Undecylenic acid | C 10 H 19 COOH | 10 | Salicornia brachiata (South Asian halophyte ) | 24.5 ° C | (10 Z ) - Undeca-10 enoic acid |
| 14: 1 | Myristoleic acid | C 13 H 25 COOH | 9 | rare fatty acid in a few vegetable oils, e.g. B. in seeds of the genus Myristicaceae ( nutmeg family ) | −4.5 ° C | (9 Z ) - tetradeca- 9-enoic acid |
| 16: 1 | Palmitoleic acid | C 15 H 29 COOH | 9 | Milk fat, animal depot fat, fish tran , vegetable fat | 1 ° C | (9 Z ) - hexadeca- 9-enoic acid |
| 17: 1 | Margaroleic acid | C 16 H 29 COOH | 9 | Animal depot fat, vegetable fats | 11.4-12.2 ° C, 14.5 ° C | (9 Z ) -Heptadeca- 9-enoic acid |
| 18: 1 | Petroselinic acid | C 17 H 33 COOH | 6th | in coriander oil ( real coriander ) | 29.8 ° C | (6 Z ) - Octadeca- 6 enoic acid |
| 18: 1 | Oleic acid (OA) | C 17 H 33 COOH | 9 | in all natural fats | 16 ° C | (9 Z ) - Octadeca- 9-enoic acid |
| 18: 1 | Elaidic acid 1 | C 17 H 33 COOH | 9 | in the fat of ruminants | 44-45 ° C | (9 E ) - Octadeca- 9-enoic acid |
| 18: 1 | Vaccenic acid | C 17 H 33 COOH | 11 | in the fat of ruminants | 44 ° C ( trans ), 14.5-15.5 ° C ( cis ) | (11 E ) (11 Z ) - Octadeca-11 enoic acid |
| 20: 1 | Gadoleic acid | C 19 H 37 COOH | 9 | Vegetable oils, cabbage species ( Brassica ); Rape and broccoli seed oil , mustard oil , fish oils | 24.5 ° C | (9 Z ) - Eicosa- 9-enoic acid |
| 20: 1 | Gondo acid | C 19 H 37 COOH | 11 | in jojoba oil, soap tree plants (Sapindaceae), cabbage species ( Brassica ), rapeseed oil (old varieties), camelina oil | 24 ° C | (11 Z ) - Eicosa-11-enoic acid |
| 22: 1 | Cetoleic acid | C 21 H 41 COOH | 11 | Vegetable oils, fish oils | 32-33 ° C | (11 Z ) - docosa-11-enoic acid |
| 22: 1 | Erucic acid | C 21 H 41 COOH | 13 | Rapeseed oil (old varieties), mustard oil | 33.5 ° C | (13 Z ) - Docosa-13-enoic acid |
| 24: 1 | Nervonic acid | C 23 H 45 COOH | 15th | Seed oil from the rare Malania oleifera tree in the Olacaceae family | 42-43 ° C | (15 Z ) - tetracosa-15-enoic acid |
| Polyunsaturated fatty acids | ||||||
|---|---|---|---|---|---|---|
| Number of carbon atoms: double bonds | Common name | Gross formula | Position of the double bonds |
Occurrence | Melting point | Chemical name |
| 18: 2 | Linoleic acid (LA) | C 17 H 31 COOH | 9.12 | Vegetable oils, especially safflower oil , sunflower oil and grape seed oil | −7 ° C | (9 Z , 12 Z ) - Octadeca- 9,12- dienoic acid |
| 18: 3 | Alpha-Linolenic Acid 2 (ALA) | C 17 H 29 COOH | 9.12.15 | some vegetable oils, especially linseed oil , walnut oil , hemp oil , rapeseed oil, and soybean oil | −11 ° C | (9 Z , 12 Z , 15 Z ) - Octadeca- 9,12,15-trienoic acid |
| 18: 3 | Gamma Linolenic Acid 2 (GLA) | C 17 H 29 COOH | 6,9,12 | in a few vegetable oils such as borage oil , evening primrose oil and hemp oil | −11 ° C | (6 Z , 9 Z , 12 Z ) - Octadeca- 6,9,12- trienoic acid |
| 18: 3 | Calendulic acid | C 17 H 29 COOH | 8,10,12 | Main fatty acid in the fatty vegetable seed oil of the marigold | 40.5 ° C | (8 E , 10 E , 12 Z ) - Octadeca- 8,10,12-trienoic acid |
| 18: 3 | Punicic acid | C 17 H 29 COOH | 9.11.13 | in a few vegetable oils, e.g. B. in the seed oil of the pomegranate | 43-44 ° C | (9 Z , 11 E , 13 Z ) - Octadeca- 9,11,13-trienoic acid |
| 18: 3 | Alpha eleostearic acid | C 17 H 29 COOH | 9.11.13 | in a few vegetable oils, e.g. B. Main fatty acid in the oil of the seeds of bitter melon ( Momordica spp.) And golden plum family ( Parinari spp.), As well as in tung oil ( Vernicia spp.) | 49 ° C | (9 Z , 11 E , 13 E ) - Octadeca- 9,11,13-trienoic acid |
| 18: 3 | Beta-eleostearic acid | C 17 H 29 COOH | 9.11.13 | in a few vegetable oils, from the α -leostearic acid in the seed oils by isomerization | 71.5 ° C | (9 E , 11 E , 13 E ) - Octadeca- 9,11,13-trienoic acid |
| 18: 4 | Stearidonic acid | C 17 H 27 COOH | 6,9,12,15 | Boraginaceae (Boraginaceae), primrose ( Primula spp.), Algae, fungi, spirulina , oils from marine animals, hemp seed, blackcurrant seed | −57 ° C | (6 Z , 9 Z , 12 Z , 15 Z ) - Octadeca- 6,9,12,15-tetraenoic acid |
| 20: 4 | Arachidonic acid | C 19 H 31 COOH | 5,8,11,14 | Animal fats, fish oil | −49.5 ° C | (5 Z , 8 Z , 11 Z , 14 Z ) - Eicosa- 5,8,11,14- tetraenoic acid |
| 20: 5 | Eicosapentaenoic acid (timnodonic acid, EPA) | C 19 H 29 COOH | 5,8,11,14,17 | Fish oils | −53–54 ° C | (5 Z , 8 Z , 11 Z , 14 Z , 17 Z ) - Eicosa- 5,8,11,14,17- pentaenoic acid |
| 22: 2 | Docosadienoic acid | C 21 H 39 COOH | 13.16 | Cod liver oil , sunflower oil , rapeseed oil (old varieties) | (13 Z , 16 Z ) - docosa-13,16-dienoic acid | |
| 22: 4 | Docosatetraenoic acid (adrenic acid, ADA) | C 21 H 35 COOH | 7,10,13,16 | Fish oils | (7 Z , 10 Z , 13 Z , 16 Z ) - docosahexaenoic acid tetra 7,10,13,16- | |
| 22: 5 | Docosapentaenoic acid , ( Clupa (no) donsäure ), (DPA-3) | C 21 H 33 COOH | 7,10,13,16,19 (4, 8, 12, 15, 19; 22: 5n-3) (4, 7, 10, 13, 16; 22: 5n-6, osbond acid , DPA-6, OBA ) |
Fish oils | −78 ° C | (7 Z , 10 Z , 13 Z , 16 Z , 19 Z ) - docosahexaenoic 7,10,13,16,19- pentanoic acid |
| 22: 6 | Docosahexaenoic acid (cervonic acid, clupanodonic acid, DHA) | C 21 H 31 COOH | 4,7,10,13,16,19 | Fish oils | −44 ° C | (4 Z , 7 Z , 10 Z , 13 Z , 16 Z , 19 Z ) - docosahexaenoic 4,7,10,13,16,19- hexaenoic |
| 24: 6 | Tetracosahexaenoic acid (nisic acid) | C 23 H 35 COOH | 6,9,12,15,18,21 | Fish oils | (6 Z 9 Z , 12 Z , 15 Z , 18 Z , 21 Z ) -Tetracosa-6,9,12,15,18,21- hexaenoic | |
- Remarks
- 1 Elaidic acid, the trans isomer of oleic acid, is produced during fat hardening for the production of margarine through the partial hydrogenation of polyunsaturated fatty acids in the course of isomerization . It occurs naturally in the fat of ruminants (milk, butter, beef tallow), as their rumen organisms also contain hydrogenating enzymes.
- 2 The linolenic acid isomer with the double bonds in positions 9, 12 and 15 (all in cis - configuration ) is alpha-linolenic acid , the isomer with the double bonds in positions 6, 9 and 12 (all in cis - Configuration) is called gamma-linolenic acid .
More fatty acids
| Fatty acids with other functional groups or special bonds | ||||||
|---|---|---|---|---|---|---|
| Number of carbon atoms: functional group double bonds |
Common name | Gross formula | Position of the functional groups / bonds |
Occurrence | Melting point |
Chemical name |
| 8: 0cy-Dis disulfide - |
Lipoic acid | C 8 H 14 S 2 O 2 | 5 (1,2- dithiolane , disulfide bridge ) | Microorganisms, algae, liver | 46-48 ° C | 5 - [(3 R ) -1,2-dithiolan-3-yl] pentanoic acid |
| 18: 0-9Br, 10Br, 12Br, 13Br bromine - ( bromoalkane , haloalkane ) |
Tetrabromostearic acid | C 18 H 32 Br 4 O 2 | 9,10,12,13 (bromo group) | Seed oil from Labiatae ( Eremostachys molucelloides ) | 114.7-115.2 ° C | 9,10,12,13-tetrabromooctadacanoic acid |
| 18: 0-9Cl-10Cl chlorine - ( chloroalkane , haloalkane) |
Dichlorostearic acid | C 18 H 34 Cl 2 O 2 | 9.10 (chlorine group) | European eel ( Anguilla anguilla ) | 9,10-dichloroctadecanoic acid | |
| 18: 1cy (18: 1-13-cp) cyclopentene - |
Chaulmoogric acid | C 18 H 32 O 2 | 13 (cyclopentene) | Seed oil Hydnocarpus wightianus , Hydnocarpus kurzii | 68.5 ° C-71 ° C | 13-Cyclopent-2-enyl-tridecanoic acid |
| 18: 1-delta-6a triple bond |
Tariric acid | C 18 H 32 O 2 | 6 (triple bond) | Vegetable oils | 49-50 ° C | 6-octadecinic acid |
| 18: 1-delta-9c-12,13-O epoxy |
Vernolic acid | C 18 H 32 O 3 | 9 (double bond) 12.13 ( epoxy group ) |
Main fatty acid in vernonia oil (from seeds of sham asters ) | 23-25 ° C | (12 R , 13 S ) -12,13-epoxy-9-cis-octadecenoic acid |
| 18: 1-delta-9c-12-OH hydroxy |
Ricinoleic acid | C 18 H 34 O 3 | 9 (double bond) 12 ( hydroxy group ) |
Main fatty acid in castor oil | 5 ° C | (9 Z , 12 R ) -12-hydroxy-9-octadecenoic acid |
| 18: 1-delta-9c-18-F fluorine - ( fluoroalkane , haloalkane) |
Fluorooleic acid | C 18 H 33 FO 2 | 9 (double bond) 18 (fluorine group) |
Seeds of Dichapetalaceae ( Dichapetalum toxicarium ) | 13.5 ° C | (9 Z ) -18-fluoro-9-octadecenoic acid |
| 18: 2-delta-5.6allene allenes - |
Laballenic acid | C 18 H 32 O 2 | 5.6 ( cumulative double bond ) | In species of mint Lamiaceae | ( R ) -5,6-octadecadienoic acid | |
| 18: 3-delta-4-oxo-9c, 11t, 13t double bond, carbonyl - |
α- licanic acid (couepic acid) | C 18 H 28 O 3 | 9,11,13 (double bond) 4 ( keto group ) keto acid |
Main fatty acid in oiticica oil (from the seeds of Licania rigida ) | 74-75 ° C | 4-oxo (9 Z , 11 E , 13 E ) octadecatrienoic acid |
| 18: 3-delta-9a, 11a, 17-E triple bond, ethene - |
Isanoic acid | C 18 H 26 O 2 | 9.11 (triple bond) 17 ( vinyl group ) |
Isano oil (from the seeds of Ongokea gore ) | 42 ° C | Octadeca-17-en-9,11-diyric acid |
| 18: 3-9-Oxa-8t, 10t, 12c double bond, vinyl ether - |
Colnelic acid | C 18 H 30 O 3 | 8,10,12 (double bond) 9 ( ether group ) |
Plant leaves and roots | (8 E ) -9 - [(1 E , 3 Z ) -1,3-nonadien-1-yloxy] -8-nonenoic acid | |
| 18: 4-delta-9c, 11t, 13t, 15c double bond |
α- parinaric acid (octadecatetraenoic acid) | C 18 H 28 O 2 | 9,11,13,15 (double bond) | Rosaceae seed oil , Balsaminaceae | 83.5 ° C | (9 Z , 11 E , 13 E , 15 Z ) -Octadecatetraenoic acid |
| 18: 4-delta-9a, 11a, 13a, 15a-17-OH triple bond, hydroxy |
Minquartinic acid | C 18 H 20 O 3 | 9,11,13,15 (triple bond) 17 (hydroxy group) |
Bark of Minquartia guianensis , Coula edulis | 97 ° C | ( S ) -17-Hydroxy-9,11,13,15-octadecatetrainic acid |
| 19: 0-11,12-cpa cyclopropyl - |
Lactobacillic acid | C 19 H 36 O 2 | 11.12 (cyclopropane) | Important fatty acid in Lactobacillus species | 28-29 ° C | (11 R , 12 S ) -Methylene octadecanoic acid |
| 19: 0-2,6,10,14-tetra-Me (19: 0br4) methyl- |
Pristanoic acid | C 19 H 38 O 2 | 2,6,10,14 ( methyl group ; isoprenoid ) | Animal fat, milk fat, fish oil | (2 S , 6 R , 10 R ) -2,6,10,14-tetramethylpentadecanoic acid | |
| 19: 1-9,10-cpe cyclopropene - |
Sterculic acid | C 19 H 36 O 2 | 9.10 (cyclopropene) | In plant seeds; Stink tree ( Sterculia foetida ), Kapok tree ( Ceiba pentandra ), African baobab ( Adansonia digitata ) |
18.2-18.3 ° C | 8- (2-octylcyclopropen-1-yl) octanoic acid |
Fatty acids with a hydroxy group are found in the lipids of animals, plants and prokaryotes. The hydroxyl group is often found on the second carbon atom (compare α- hydroxycarboxylic acids ). Β-hydroxy fatty acids also occur, as do fatty acids in which the functional group occurs in the middle of the carbon chain, such as ricinoleic acid. Further functional groups with an oxygen atom are the epoxy group , the keto group and the furan group , which can also be found in fatty acids.
Fatty acids in the membrane lipids of bacteria sometimes have unusual components in the molecule. So have alicyclic fatty acids on a ring of hydrocarbons. As cyclopropane , this can be in the middle of the carbon chain, as is the case with mycolic acids or lactobacillic acid . Furthermore, they can also have a keto group. Mycolic acids are also the longest naturally occurring fatty acids. They are bound to the murein in the bacterial cell wall via arabinogalactan .
Rings with six or seven carbon atoms ( cyclohexane or cycloheptane ) are often found at the end of the actual fatty acid chain; they are then referred to as omega-alicyclic (ω-alicyclic) fatty acids, with the Greek lowercase ω being used as locant . The bacterial genus Alicyclobacillus was named after these fatty acids because it contains them in large quantities in the membrane lipids. One example is omega-cyclohexyltridecanoic acid, an ω-alicyclic fatty acid with a cyclohexane residue and a chain with 13 carbon atoms.
metabolism
transport
Fatty acids are stored as triglycerides in adipose tissue. If necessary, indicated by the messenger substances adrenaline , noradrenaline , glucagon or ACTH , lipolysis takes place there.
The free fatty acids are then transported in the bloodstream to the energy-consuming cells, where they are first bound (activated) to coenzyme A (CoA) while consuming ATP . This reaction is driven by the hydrolysis of the resulting pyrophosphate to two phosphates (P i ).
Then they are bound to carnitine by the enzyme carnitine acyltransferase I and actively transported into the matrix of the mitochondria , where they are bound to CoA again by carnitine acyltransferase II. This activation is necessary because fatty acids can diffuse through the mitochondrial membrane. Only actively transported fatty acids are used for the β-oxidation of the fatty acids. The acyl carnitine activation is not reversible, an activated fatty acid is broken down.
Fatty acid breakdown
In the matrix of the mitochondrion, the β-oxidation of the fatty acids to acetyl-CoA takes place, which can be used in the citric acid cycle to gain ATP. For prolonged periods of hunger or diets with very little carbohydrates , such as B. the Atkins diet , the fats are instead metabolized into ketone bodies .
In addition to the mitochondrial fatty acid oxidation, fatty acids are also utilized in the peroxisomes . Especially very long-chain fatty acids are usually shortened there first before they can be processed further in the mitochondria. This peroxisomal function is significant. Failure will lead to adrenoleukodystrophy .
Fatty acid synthesis
In contrast to its breakdown, fatty acid synthesis takes place in the cytosol . In higher organisms, all of the enzymes required for this are combined in a single enzyme complex, the fatty acid synthase . In green plants, however, the synthesis up to the C18 fatty acid takes place mainly in the plastids and is only then transported into the cytosol.
For this purpose, malonyl-CoA is first formed from acetyl-CoA with consumption of ATP by carboxylation . This is then converted to malonyl-ACP, because in contrast to the breakdown, acyl carrier protein (ACP) serves as a carrier molecule instead of CoA. The subsequent condensation reaction is roughly a reversal of fatty acid oxidation ( β-oxidation ). However, there are some significant differences in the details that allow independent, targeted control of both processes.
Characteristic fatty acids in microorganisms
Typical fatty acids can be used as biomarkers . Actinomycetes are Gram-positive bacteria that occur during the decomposition of organic material and, among other things, produce an earthy odor. Actinomycete fatty acids are occasionally branched at C10 with a methyl group, e.g. B. 16: 0 10-methyl and 18: 0 10-methyl. Actinomycetes living in the ground are e.g. B. Rhodococcus, Nocardia, Corynebacterium and Streptomyces . Gram-positive bacteria are e.g. B. also Bacillus spp. such as Bacillus cereus and Bacillus subtilis . The number of bacteria of Bacillus spp. increases in the rhizosphere . They form branched fatty acids like 15: 0 iso and 15: 0 anteiso.
Gram-negative bacteria are an important part of the rhizosphere and increase the availability of phosphate, iron and other minerals, some also produce fungicides . Gram-negative bacteria produce higher concentrations of monounsaturated fatty acids such as 16: 1 omega-7 and 18: 1 omega-9, most of which are further metabolized into cyclopropyl fatty acids such as 17: 0 cyclopropane and 19: 0 cyclopropane. Dimethylacetals (DMA) are formed under anaerobic conditions and can be used as biomarkers. Under strictly anaerobic conditions, such as during a flood, the number of facultative aerobic bacteria decreases and the number of anaerobic bacteria and archaea increases.
The fatty acids in the lipids of archaea are not linked by an ester bond , but by an ether bond . Mycorrhizal fungi form storage vesicles that contain , among other things, 18: 2 (ω-6c) and 16: 1 (ω-5c).
Different fatty acids as biomarkers:
- Even-numbered fatty acids (e.g. 16: 0, palmitic acid ) - prokaryotes and eukaryotes
- iso -branched fatty acids (e.g. 17: 0 iso, 15-methylpalmitic acid) - Gram-positive bacteria
- anteiso -branched fatty acids (e.g. 17: 0 anteiso, 14-methylpalmitic acid) - Gram-positive bacteria
- 10-methyl-branched fatty acids (e.g. 19: 0 10-methyl, tuberculostearic acid ) - Actinomycetales
- Monounsaturated fatty acids (Engl. Monounsaturated fatty acids , MUFA)
- 16: 1 ω5c (hexadecenonic acid) - mycorrhiza
- Omega-5 and 7 position (e.g. 16: 1 ω7c, palmitoleic acid ) - Gram-negative bacteria
- 16: 1 ω8c (8-hexadecenonic acid) - methane-oxidizing bacteria type I
- 18: 1 ω8c (10-octadecenonic acid) - methane-oxidizing bacteria type II
- Omega-9 position (e.g. 16: 1 ω9c, cis-7-palmitoleic acid) - Ectomycorrhizal fungi & Gram-positive bacteria
- Polyunsaturated fatty acids (Engl. Polyunsaturated fatty acids , PUFA)
- 18: 2ω6c, ( linoleic acid ) - ectomycorrhizal fungi
- 20: 2 ω6c, 20: 3 ω6c, 20: 4 ω6c - protozoa
- Other PUFA - eukaryotes
- Cyclopropyl fatty acids (e.g. 19: 0 cyclo ω7c) - bacteria
- Dimethyl acetals (e.g. 16: 0 DMA, hexadecanal dimethyl acetal) - Anaerobic bacteria
Health importance
Both saturated and unsaturated fatty acids provide a lot of energy, support the immune system, and reduce a. Depression and have a positive effect on many other metabolic processes. Fats with a high percentage of medium-chain fatty acids are easier to digest than those with long-chain fatty acids.
In an evaluation of intervention studies with over 13,600 participants in 2010, the German Nutrition Society (DGE) found that a high proportion of polyunsaturated fatty acids, together with a low proportion of saturated fatty acids, increased the risk of coronary heart disease (e.g. heart attack ) lowers. It confirmed results that Daniel and Hecht published in 1990. Favorable ratios of polyunsaturated to saturated fatty acids are found primarily in vegetable fats (e.g. safflower oil 74.5% / 8.6%, olive oil 86% / 14%, hemp oil 70% / 10%, sunflower oil 60.7% / 11 , 5% and soybean oil 61.0% / 13.4%; exceptions are coconut oil 1.4% / 86.5% and palm oil 17% / 83%), nuts and seeds (e.g. tahini 55% / 9% , Hazelnuts 54% / 5%).
Unsaturated trans fatty acids have a negative effect on cholesterol levels . In particular, the lowering of the HDL cholesterol level with a simultaneous increase in the LDL cholesterol lipoprotein (a) level and proinflammatory effects have a negative impact on the endothelial function of the arterial walls. There are also suspicions of an increase in insulin resistance and obesity , cell membrane changes and negative effects on blood clotting. In addition, the evidence from observational studies for a connection between trans fatty acids and an increased risk of coronary heart disease is very convincing. Foods with triglycerides containing trans fatty acids are often marked with the note “vegetable oil, partially hydrogenated” in the information on the ingredients .
In populations in the Mediterranean region, the intake of monounsaturated fatty acids is between 16 and 29% of the total daily energy intake (mainly in the form of oleic acid, e.g. olive oil ). Studies show that replacing saturated fatty acids with carbohydrates, monounsaturated or polyunsaturated fatty acids reduces cardiovascular risk factors. Compared to carbohydrates, MUFAs had a positive effect on triglycerides, HDL cholesterol and the total cholesterol: HDL cholesterol ratio. Two meta-analyzes showed positive effects of an increased intake of monounsaturated fatty acids on the following cardiovascular risk factors: systolic and diastolic blood pressure , glycated hemoglobin ( HbA1c ) and fasting glucose .
The omega-6 fatty acids (e.g. linoleic acid , gamma-linolenic acid ) and the omega-3 fatty acids belong to the essential fatty acids, as they cannot be produced by the human organism itself. In vegetable oils, linoleic acid (sunflower oil, soybean oil, corn oil) occurs in very high concentrations (50–70% based on the total fatty acid content). Through dehydration and chain extension, the human organism can convert linoleic acid through several intermediate stages to arachidonic acid . Arachidonic acid can be further converted to prostaglandins in the body. Linseed and hemp oil are rich in linolenic acid, arachidonic acid is only found in animal products such as liver, eggs and lard . The essential fatty acids are involved in the construction of cell membranes and lower blood fat and cholesterol levels.
Omega-6 fatty acids are mostly metabolized via arachidonic acid - but not always or exclusively - into prostaglandins that promote inflammation , while omega-3 fatty acids become anti-inflammatory.
The DGE recommends covering around 30% of the energy requirement with fat. 10% should be covered with saturated fatty acids, 10 to 13% with monounsaturated and the rest with polyunsaturated. The American Heart Society (ADA), the European Food Safety Authority (EFSA) and the American Academy of Nutrition and Dietetics recommend that less than 35% of the energy requirement be obtained from fat, with the ADA an energy intake of less than 20% from monounsaturated fatty acids recommends. To keep the cardiovascular risk low, the ratio of omega-6 to omega-3 fatty acids should be a maximum of 5: 1. An international commission of experts headed by Berthold Koletzko ( Children's Health Foundation ) has developed and published guidelines for the nutrition of mothers and babies. It describes that the growing fetus increasingly needs long-chain, polyunsaturated fatty acids, so-called LC-PUFA (Longchain polyunsaturated fatty acid). In particular, these are arachidonic acid (omega-6 fatty acid, AA) and docosahexaenoic acid (omega-3 fatty acid, DHA). The fatty acids mentioned are contained in fatty sea fish (e.g. herring , mackerel and salmon).
Substituted fatty acids with keto and hydroxy groups are present in spoiled oils. Some of them are poisonous for the human organism. Another important substituted fatty acid, ricinoleic acid , is about 80% in castor oil . Castor oil is not absorbed in the intestine and therefore has a laxative effect.
Analysis of fatty acids
The modern qualitative and quantitative analysis of fatty acids in food chemistry and in physiological research usually makes use of chromatographic methods. Capillary gas chromatography (after transesterification to the methyl ester), HPLC and the coupling of these processes with mass spectrometry are used . Usually the fatty acids are in the form of suitable derivatives , such as. B. the fatty acid methyl esters or their TMS derivatives, separated by chromatography. In special cases, classic column and thin-layer chromatography is still used today ; the isomers are separated by means of silver nitrate thin layer chromatography.
Count of fatty acids
The number of unbranched fatty acids (including shorter monocarboxylic acids) with different numbers of double bonds at different positions as a function of chain length obeys the Fibonacci sequence, which is very well known in number theory . Among other things, this follows from the fact that (with rare exceptions) there are no neighboring double bonds in fatty acids. Specifically, there is only one aliphatic monocarboxylic acid with one carbon atom: formic acid, one with two carbon atoms: acetic acid, two with three: propionic acid and acrylic acid , etc. With 18 carbon atoms there are 2,584 variants (of which stearic acid, oleic acid, linoleic acid and Linolenic acid are four examples).
Special fatty acids
- Furan fatty acids
- Free fatty acids
- Lipoic acid (sulphurous fatty acid)
literature
- Wolf-H. Kunau: chemistry and biochemistry of unsaturated fatty acids. In: Angewandte Chemie . 88, 1976, pp. 97-111 ( doi: 10.1002 / anie.19760880402 ).
- J. Ernst, WS Sheldrick, J.-H. Fuhrhop: The structures of the essential unsaturated fatty acids. Crystal structure of linoleic acid as well as evidence for the crystal structures of linolenic acid and arachidonic acid. In: Z. Naturforsch. 34b, 1979, pp. 706-711.
- P. Nuhn , M. Gutheil, B. Dobner: Occurrence, biosynthesis and importance of branched fatty acids. In: Fette-Soap-Paint. 87, 1985, p. 135.
- FD Gunstone, JL Harwood, FB Padley: The Lipid Handbook. Chapman and Hall, London / New York 1986, ISBN 0-412-24480-2 .
Web links
- Common (Nonsystematic) Names for Fatty Acids (PDF; 196 kB), from AOCS , accessed on October 20, 2017.
- PlantFA Database , accessed May 24, 2017.
- Botany online: lipids at biologie.uni-hamburg.de.
- Fatty acid composition of important vegetable and animal edible fats and oils on dgfett.de.
Individual evidence
- ↑ Entry on fatty acids . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.F02330 Version: 2.3.1.
- ↑ T. Rezanka, K. Sigler: Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. In: Progress in lipid research. Volume 48, number 3-4, 2009, pp. 206-238, doi: 10.1016 / j.plipres.2009.03.003 , PMID 19336244 .
- ↑ Michael T. Madigan, John M. Martinko, Jack Parker: Brock Microbiology. German translation edited by Werner Goebel, Spektrum Akademischer Verlag, Heidelberg / Berlin 2000, ISBN 3-8274-0566-1 , pp. 500–501.
- ↑ P. Pohl, H. Wagner: Fatty acids in the plant and animal kingdom (an overview). In: fats, soaps, paints. 74, 1972, pp. 424-435 and 542-550.
- ↑ Gebauer, Psota, Kris-Etherton: The diversity of health effects of individual trans fatty acid isomers. In: Lipids. Volume 42 (9), 2007, pp. 787-799, doi: 10.1007 / s11745-007-3095-8 .
- ↑ Toshi Kaneda: Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. In: Microbiological reviews. Volume 55, Number 2, 1991, pp. 288-302, PMID 1886522 , PMC 372815 (free full text), (review).
- ↑ Hans-Dieter Belitz, Walter Grosch: Textbook of food chemistry . 4th edition. Springer Verlag, Heidelberg / Berlin 1992, ISBN 3-540-55449-1 , p. 465-466 .
- ^ William W. Christie: Fatty Acids: Branched-chain - Structure, Occurence and Biosynthesis. (No longer available online.) In: AOCS Lipid Library website . June 26, 2012, archived from the original on January 12, 2010 ; accessed on March 8, 2014 .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-4 - 3-523.
- ↑ Entry on palmitic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ^ A b c Albin H. Warth: The Chemistry and Technology of Waxes. Second Edition, Reinhold Publ., 1956, p. 34, online at babel.hathitrust.org, accessed November 1, 2017.
- ↑ a b Shmuel Yannai: Dictionary of Food Compounds. Second Edition, CRC Press, 2012, ISBN 978-1-4200-8351-4 , pp. 883, 1023.
- ↑ a b c H. M. Rauen: Biochemisches Taschenbuch. Springer, 1956, ISBN 978-3-642-53241-2 (reprint), pp. 162, 232.
- ^ The Seed Oil Fatty Acids (SOFA) database .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 97th edition. (Internet version: 2016), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-338.
- ↑ Reinhard Lieberei, Christoph Reisdorff: Useful plants. 8th edition, Thieme, 2012, ISBN 978-3-13-530408-3 , p. 137.
- ↑ Wolfgang Steglich, Burkhard Fugmann: RÖMPP Lexikon Naturstoffe. Thieme, 1997, ISBN 3-13-749901-1 , p. 142.
- ^ John W. Blunt, Murray HG Munro: Dictionary of Marine Natural Products. Chapman & Hall, 2008, ISBN 978-0-8493-8216-1 , pp. 701 f.
- ^ J. Elks, CR Ganellin: The Dictionary of Drugs. Springer, 1990, ISBN 978-1-4757-2087-7 , p. 734.
- ^ Dictionary of Organic Compounds. Second Supplement, Sixth Edition, Chapman & Hall, ISBN 978-0-412-54120-9 , p. 260.
- ↑ Frank D. Gunstone, John L. Harwood, Albert J. Dijkstra: The Lipid Handbook. Third Edition, CRC Press, 2007, ISBN 0-8493-9688-3 , p. 12.
- ↑ R. Hegnauer: Chemotaxonomy of plants. Volume 4, Springer, 1966, ISBN 978-3-0348-9383-1 , p. 158.
- ↑ W. Ruhland (Ed.): Handbuch der Pflanzenphysiologie. Volume 7, Springer, 1957, ISBN 978-3-642-94705-6 , p. 20.
- ↑ W. Karrer: Constitution and occurrence of organic plant matter. Supplementary volume 1, Birkhäuser, 1977, ISBN 978-3-0348-9378-7 , p. 338.
- ↑ a b W. Karrer : Constitution and occurrence of organic plant matter. 2nd edition, Springer, 1976, ISBN 978-3-0348-5143-5 (reprint), pp. 317, 395.
- ^ William W. Christie: Fatty Acids: Hydroxy and other oxygenated - Structures, Occurence and Biochemistry. (No longer available online.) In: AOCS Lipid Library website . October 29, 2013, archived from the original on December 10, 2009 ; accessed on March 8, 2014 .
- ^ DW Grogan, JE Cronan: Cyclopropane ring formation in membrane lipids of bacteria. In: Microbiology and molecular biology reviews: MMBR. Volume 61, Number 4, 1997, pp. 429-441, PMID 9409147 , PMC 232619 (free full text). (Review).
- ↑ H. Matsubara, K. Goto et al. a .: Alicyclobacillus acidiphilus sp. nov., a novel thermo-acidophilic, omega-alicyclic fatty acid-containing bacterium isolated from acidic beverages. In: International journal of systematic and evolutionary microbiology. Volume 52, No. 5, 2002, pp. 1681-1685. PMID 12361274 .
- ^ L. Zelles: Identification of single cultured micro-organisms based on their whole-community fatty acid profiles, using an extended extraction procedure. In: Chemosphere. Volume 39, Number 4, August 1999, pp. 665-682. PMID 10422254 .
- ↑ A. Frostegård, A. Tunlid, E. Bååth: Phospholipid Fatty Acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. In: Applied and Environmental Microbiology . Volume 59, Number 11, November 1993, pp. 3605-3617. PMID 16349080 . PMC 182506 (free full text).
- ↑ A. Pandey, P. Trivedi, B. Kumar, LM Palni: Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a sub-alpine location in the Indian Central Himalaya. In: Current microbiology. Volume 53, Number 2, August 2006, pp. 102-107, doi: 10.1007 / s00284-006-4590-5 . PMID 16832725 .
- ↑ A. Kaur et al. a .: Phospholipid fatty acid - A bioindicator of environmental monitoring and assessment in soil ecosystem. In: Current Science. Volume 89, Issue 7, 2005, pp. 1103-1112.
- ↑ L. Zelles: Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: a review. In: Biol Fertil Soils. Volume 29, Issue 2, 1999, pp. 111-129.
- ^ Q. Bai, A. Gattinger, L. Zelles: Characterization of Microbial Consortia in Paddy Rice Soil by Phospholipid Analysis. In: Microbial ecology. Volume 39, Number 4, May 2000, pp. 273-281. PMID 10882432 .
- ↑ J. Lombard, P. López-García, D. Moreira: Phylogenomic investigation of phospholipid synthesis in archaea. In: Archaea (Vancouver, BC). Volume 2012, 2012, p. 630910, doi: 10.1155 / 2012/630910 , PMID 23304072 , PMC 3533463 (free full text).
- ^ IM van Aarle, PA Olsson: Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. In: Applied and Environmental Microbiology . Volume 69, Number 11, November 2003, pp. 6762-6767, PMID 14602638 , PMC 262256 (free full text).
- ↑ Polyunsaturated fatty acids lower the risk of coronary heart disease (PDF; 240 kB).
- ^ H. Daniel, H. Hecht: Nutrition and Arteriosclerosis . In: Deutsche Apotheker Zeitung . 1990, p. 1307-1318 .
- ^ A b Claus Leitzmann , Andreas Hahn: Vegetarian Diet . 1st edition. Ulmer, Stuttgart 1996, ISBN 3-8252-1868-6 , pp. 88, 89 .
- ↑ Entry on olive oil. In: Römpp Online . Georg Thieme Verlag, accessed on July 24, 2013.
- ↑ R. Uauy, A. Aro, R. Clarke, Ghafoorunissa, MR L'Abbé, D. Mozaffarian, CM Skeaff, p Stender, M. Tavella: WHO Scientific Update on trans fatty acids: summary and conclusions . In: European Journal of Clinical Nutrition . tape 63 , S2, 2009, pp. S68 – S75 , doi : 10.1038 / ejcn.2009.15 .
- ↑ Renata Micha, Dariush Mozaffarian: Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes . In: Nature Reviews Endocrinology . tape 5 , no. 6 , June 2009, p. 335–344 , doi : 10.1038 / nrendo.2009.79 .
- ↑ D. Mozaffarian, A. Aro, WC Willett: Health effects of trans-fatty acids: experimental and observational evidence . In: European Journal of Clinical Nutrition . tape 63 , S2, January 2009, pp. S5-S21 , doi : 10.1038 / sj.ejcn.1602973 .
- ↑ C. Murray Skeaff, Jody Miller: Dietary Fat and Coronary Heart Disease: Summary of Evidence from Prospective Cohort and Randomized Controlled Trials . In: Annals of Nutrition and Metabolism . tape 55 , no. 1-3 , September 2009, pp. 173-201 , doi : 10.1159 / 000229002 .
- ↑ Marcel Kollmar (2012): Saturated and unsaturated fatty acids. Which fats does the body need? on joggen-online.de.
- ↑ The Joint FAO / WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition November 10–14, 2008, Geneva Switzerland (2010): Interim summary of conclusions and dietary recommendations on total fat and fatty acids. (PDF; 1.9 MB).
- ↑ L. Schwingshackl, B. Strasser, G. Hoffmann: Effects of monounsaturated fatty on glycemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. In: Ann Nutr Metab. 58, 2011, pp. 290-296, doi: 10.1159 / 000331214 .
- ^ L. Schwingshackl, B. Strasser, G. Hoffmann: Effects of Monounsaturated Fatty Acids on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. In: Ann Nutr Metab. 59, 2011, pp. 176-186, doi: 10.1159 / 000334071 .
- ↑ L. Schwingshackl, B. Strasser: High-MUFA Diets Reduce Fasting Glucose in Patients with Type 2 Diabetes. In: Ann. Nutr. Metab. 60, 2012, pp. 33-34, doi: 10.1159 / 000335162 .
- ↑ Nutrition, and Allergies (NDA) EFSA Panel on Dietetic Products: Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol . In: EFSA Journal . tape 8 , no. 3 , March 2010, doi : 10.2903 / j.efsa.2010.1461 .
- ^ American Dietetic Association, Dietitians of Canada: Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. In: J. Am. Diet Assoc. 107 (9), 2007, pp. 1599-1611, PMID 17936958 .
- ↑ American Heart Association Nutrition Committee: Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. In: Circulation. 114 (1), 2006, pp. 82-96. PMID 16785338 .
- ↑ Berthold Koletzko, Eric Lien, Carlo Agostoni a. a .: The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations . In: Journal of Perinatal Medicine . tape 36 , no. 1 , 2008, p. 5-14 , doi : 10.1515 / JPM.2008.001 .
- ↑ B. Breuer, T. Stuhlfauth, HP Fock: Separation of fatty acids or methyl esters including positional and geometric isomers by alumina argentation thin-layer chromatography. In: J. of Chromatogr. Science. 25, 1987, pp. 302-306, doi: 10.1093 / chromsci / 25.7.302 .
- ^ S. Schuster, M. Fichtner, S. Sasso: Use of Fibonacci numbers in lipidomics - Enumerating various classes of fatty acids. In: Sci. Rep. 7 (2017) 39821, doi: 10.1038 / srep39821 .
- ^ HA Harper: Physiological chemistry. Springer-Verlag, 2013, ISBN 978-3-662-09766-3 , p. 595.




