Cyclopropane
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
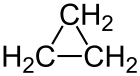
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Cyclopropane | |||||||||||||||||||||
| other names |
Trimethylene |
|||||||||||||||||||||
| Molecular formula | C 3 H 6 | |||||||||||||||||||||
| Brief description |
colorless gas with an odor similar to petroleum ether |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 42.08 g · mol -1 | |||||||||||||||||||||
| Physical state |
gaseous |
|||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point |
−127.62 ° C |
|||||||||||||||||||||
| boiling point |
−32.9 ° C |
|||||||||||||||||||||
| solubility |
very heavy in water (502 mg l −1 ) |
|||||||||||||||||||||
| Dipole moment |
0 |
|||||||||||||||||||||
| Refractive index |
1.3799 (−42 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
not fixed |
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
53.3 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Cyclopropane is a colorless, gaseous cycloalkane , the first member of this class of compounds.
discovery
In an attempt to extend the process of the Wurtz reaction ( Wurtz synthesis ) to α, ω-dihaloalkanes, August Freund discovered a hydrocarbon with the empirical formula C 3 H 6 , which he named trimethylene .
Extraction and presentation
Gustavson found a simpler, safer synthesis in 1887 by using zinc dust in hydrous ethanol for dehalogenation instead of the sodium metal that had been common up until then. Production according to this reaction principle is still the cheapest today; it has been optimized and also implemented with 1,3-dichloropropane or the easily accessible 1-bromo-3-chloropropane .
properties
Physical Properties

The molecule belongs to the symmetry group D 3h . A structure determination by electron diffraction showed a distance between the carbon atoms of 151.0 pm . A value of 108.9 pm was determined for the C – H bond length and 115.1 ° for the HCH angle.
The three carbon atoms of the cyclopropane must necessarily lie in one plane. In the classical, i.e. non- quantum chemical Baeyer model , a high angular stress is therefore to be expected, as well as torsional stress ( Pitzer stress ) due to the ecliptic position of the hydrogen atoms. Typically 117 kJ / mol (28 kcal / mol) are specified for the tension energy. The structural data show, however, that the cyclopropane must have special bonding conditions. Simple models like the Baeyer model are therefore not appropriate.
Cyclopropane cannot be understood with the concept of strictly localized sigma bonds. In the small molecule, electron interactions occur which are not important in the larger cycloalkanes. Cyclopropane therefore differs greatly from other cycloalkanes in terms of its physical and chemical properties.
Quantum chemical calculations using ab initio methods (see article Chemoinformatics ) have shown that the electron population has high values outside the edges of the hypothetical triangle, i.e. the straight line through which the centers of the C atomic nuclei can be thought of as being connected.
The physical chemist Theodor Förster was probably the first to show that in cyclopropane the "valence direction" differs from the "bond direction", i.e. H. the C – C bond in the classic formula.
Charles Coulson and WE Moffitt developed this orbital model, which was later named after them, further.
The valence orbitals of an sp 3 -hybridized carbon atom form angles of 109.4 ° and have 25% s-character and 75% p-character. Two of them are used for the bonds to the hydrogen atoms. In the case of cyclopropane, the remaining orbitals of three CH 2 fragments would have to be combined. The area of the overlap is then small and does not lie in the connecting line of the carbon atoms.
The overlap becomes greater if hybrid orbitals with a higher p component are used. The optimum was found to be an orbital which has only 17% s character for the C – C bond, but 33% s character for the C – H bonds, i.e. which could be referred to as sp 5 orbital.
This leads to the image of “bent” C – C bonds , which have been casually referred to as “ banana bonds ”. The valence angles (interior angles) of the C – C – C bonds are 104 °.
The model explains why the C – C bonds are shorter than in cyclohexane and straight-chain saturated hydrocarbons ( alkanes ) and yet weaker. The higher s-character of the C – H bonds affects the force constant of the C – H bond (infrared and Raman spectrum), the chemical shift and coupling constants in NMR spectra, and the increased CH acidity of the hydrocarbon.
A second orbital model was developed by Arthur Donald Walsh . The Walsh model is based on sp 2 hybrid orbitals for the CH 2 group; the “bent” bonds then arise from the overlap of pure p orbitals.
Cyclic delocalization of the six electrons of the three CC σ bonds of cyclopropane was used by Michael JS Dewar as an explanation for the comparatively low strain energy compared to cyclobutane ("only" 115.6 vs. 109.7 kJ / mol, reference cyclohexane with E sp = 0 kJ / mol) (stabilization through σ-aromaticity, cf. the cyclic delocalization of the six π-electrons in benzene as a prime example of aromaticity). The assumption of a diamagnetic ring current in the cyclopropane is evidenced by the reduced voltage energy in the shielding of the cyclopropane protons in the NMR and by its unusual magnetic properties (high diamagnetic susceptibility, high anisotropy of the magnetic susceptibility). More recent studies on the extent to which cyclopropane is stabilized by σ-aromaticity assign the effect a value of 11.3 kcal mol −1 .
Chemical properties
Compared to higher cycloalkanes, cyclopropane shows a special reactivity pattern: a C – C bond is often broken. Association reactions are characteristic. Explanations were given, among other things, with the concepts of " tension energy " and " Walsh model ".
When cyclopropane, which was labeled with deuterium atoms, was heated, a "geometric isomerization" was observed: cis -1,2-dideuteriocyclopropane forms trans -1,2-dideuteriocyclopropane in an equilibrium reaction.
The deuterium atoms change their position (gr. Topos ), so this process can be described as topomerization of the molecule. A concerted mechanism was discussed.
At around 500 ° C, cyclopropane is converted to propene . This constitutional isomerization, investigated in the range from 469.6 to 518.6 ° C, requires an activation energy of about 65 kcal mol −1 . A "trimethylene diradical" (propane-1,3-diyl) was postulated as an intermediate stage.
On a palladium catalyst , cyclopropane reacts with hydrogen to form propane . It reacts with hydrogen bromide (HBr) to form 1-bromopropane , which also shows its "olefinic character". Chlorine gas also reacts with addition to 1,3-dichloropropane.
use
In the past it was owned mixed with a 15-30% oxygen as a narcotic analgesic used to what today because of the disadvantages (explosiveness of the fumes , poor controllability of anesthesia , cardiac and hepatic toxicity ) apart.
safety instructions
Like all lower hydrocarbons, cyclopropane forms easily flammable mixtures with air. The explosion range is between 2.4% by volume (40 g / m 3 ) as the lower explosion limit (LEL) and 10.4% by volume (385 g / m 3 ) as the upper explosion limit (UEL). The limit gap width was determined to be 0.91 mm. This results in an assignment to explosion group IIA. With a minimum ignition energy of 0.17 mJ, vapor-air mixtures are extremely ignitable. The ignition temperature is 495 ° C. The substance therefore falls into temperature class T1.
Derivatives and substituents
Numerous derivatives can be prepared by chemical synthesis, e.g. B. with an alkyl group as the remainder. They are studied in the petroleum industry , cancer research and other scientific fields.
The biosynthesis is catalyzed by cyclopropane synthase, so that mycolic acids are formed, which are a key factor in making tuberculosis difficult to treat with hydrophobic antibiotics. The enzyme itself is a methyl transferase that uses S-adenosyl methionine to build cis or trans cyclopropane rings into the unsaturated fatty acid there.
Web links
Individual evidence
- ↑ a b c d e f g h i j Entry on cyclopropane in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-132.
- ↑ Entry on cyclopropane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
- ↑ August Freund: About trimethylene . In: Monthly books for chemistry and related parts of other sciences . Vol. 3, No. 1 (1882), pp. 625-635. doi : 10.1007 / BF01516828
- ↑ G. Gustavson: About a new method of presentation of trimethylene . In: Journal for practical chemistry . Vol. 36 (1887), pp. 300-303. doi : 10.1002 / prac.18870360127
- ↑ D. Wendisch: Carbocyclic three-ring compounds. In: Eugen Müller (ed.): Methods of Organic Chemistry (Houbel-Weyl). Vol. IV / 3, Thieme, Stuttgart 1971, pp. 34-36.
- ^ O. Bastiansen, FN Fritsch, K. Hedberg: Least-squares refinement of molecular structures from gaseous electron-diffraction sector-microphotometer data. III. Refinement of cyclopropane. In: Acta Cryst. 17, (1964), pp. 538-543. doi : 10.1107 / S0365110X64001268
- ^ A b S. W. Benson, Themochemical Kinetics, p. 273, J. Wiley & Sons, New York, London, Sydney, Toronto 1976
- ↑ Graphical representation see z. B. with Martin Klessinger: Electronic structure of organic molecules. Verlag Chemie, Weinheim u. a. 1982, ISBN 3-527-25925-2 , pp. 264-266.
- ↑ Th. Förster: The mutual influence of the valences in the carbon atom . In: Journal of Physical Chemistry . Division B, 43, 58-78 (1939).
- ^ A b C. A. Coulson, WE Moffitt: Strain in Non-Tetrahedral Carbon Atoms . In: Journal of Chemical Physics . Vol. 15, (1951), p. 151.
- ^ CA Coulson, WE Moffitt: The properties of certain strained hydrocarbons . In: Philosophical Magazine Series. 7, Vol. 40 (1949), No. 300, pp. 1-35. doi : 10.1080 / 14786444908561208
- ↑ L. Klasinc, Z. Maksić, M. Randić: Bent bonds in cyclo alkanes . In: J. Chem. Soc. A. 1966, pp. 755-757. doi : 10.1039 / J19660000755
- ^ AD Walsh: The structures of ethylene oxide, cyclopropane, and related molecules . In: Transactions of the Faraday Society . 45, (1949), pp. 179-190, doi : 10.1039 / TF9494500179
- ↑ MJ Dewar. Chemical implicatons of σ conjugation . In: J. Am. Chem. Soc. , 1984, 106, pp. 669-682.
- ↑ D. Cremer. Pros and Cons of σ-Aromaticity . In: Tetrahedron , 1988, 44 (2), pp. 7427-7454.
- ^ Kai Exner and Paul von Ragué Schleyer. Theoretical Bond Energies: A Critical Evaluation . In: J. Phys. Chem. A , 2001, 105 (13), pp. 3407-3416. doi : 10.1021 / jp004193o .
- ↑ BSRabinovitch, EW Schlag , K. Wiberg: Geometrical and Structural Unimolecular Isomerization of Sym ‐ Cyclopropane ‐ d 2 . In: Journal of Chemical Physics . 28 (1958), p. 504.
- ^ EW Schlag , BS Rabinovitch: Kinetics of the Thermal Unimolecular Isomerization Reactions of Cyclopropane-d 2 . In: Journal of the American Chemical Society . 82 (1960), No. 23, pp. 5996-6000. doi : 10.1021 / ja01508a008
- ↑ Thomas Seal Chambers, GB Kistiakowsky: Kinetics of the Thermal Isomerization of Cyclopropane . In: Journal of the American Chemical Society . 56 (1934), No. 2, pp. 399-405. doi : 10.1021 / ja01317a036
- ↑ G. Gustavson: About the action of chlorine on trimethylene. In: Journal for practical chemistry . 42, pp. 495-500 (1890). doi : 10.1002 / prac.18900420144
- ^ A b c d E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ Technical rule for operational safety - TRBS 2153, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , as of April 2009, Jedermann-Verlag Heidelberg.
- ↑ Geoffrey C. Bond: Metal-Catalysed Reactions of Hydrocarbons. Springer Science & Business Media, 2006, ISBN 978-0-387-26111-9 , p. 487 ( limited preview in Google book search).
- ^ Tanya Parish: Mycobacterium. Horizon Scientific Press, 2009, ISBN 978-1-904-45540-0 , p. 24 ( limited preview in Google book search).







