Calendulic acid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
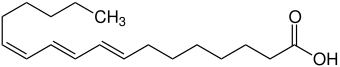
|
||||||||||
| α-calendulic acid | ||||||||||
| General | ||||||||||
| Surname | Calendulic acid | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 18 H 30 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 278.44 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Calendulic acid is an organic compound from the group of conjugated , polyunsaturated fatty acids . It is a trienoic acid in the group of trans fatty acids and belongs to the omega-6 fatty acids . The common name is derived from the plant genus of marigolds ( Calendula ), from which the natural substance esterified as triacylglyceride was isolated for the first time. It exists in three isomeric forms, which differ in the arrangement of the substituents on the double bond.
The mixed configured α- calendulic acid (8 E , 10 E , 12 Z ) (calendic acid) and the all- trans β- calendulic acid (8 E , 10 E , 12 E ) from the seed oil of the marigold ( Calendula officinalis ) and the mixed configured Jacaric acid (8 Z , 10 E , 12 Z ) from the seeds of the rosewood tree ( Jacaranda mimosifolia ) belong to the group of natural conjugated linolenic acids (CLN). They are positional isomers of linolenic acid .
Biochemically, the α- calendulic acid in Calendula officinalis is synthesized from linolate (anion of linoleic acid ) by an unusual Δ12-oleate desaturase (FAD2 variant), which converts the cis double bond of linoleic acid at position 9 into the 8- trans -, 10- converts trans- conjugated double bond system. There are also genetically modified soybeans that are used to produce calendulic acid.
Calendula oil extracted from the flowers of the marigold is used to make anti-inflammatory ointments; From the oil of the seeds, which contains approx. 60% esterified calendulic acid, varnishes and varnishes are made.
Individual evidence
- ↑ Shmuel Yannai: Dictionary of Food Compounds. Second Edition, CRC Press, 2012, ISBN 978-1-4200-8351-4 , p. 1492.
- ↑ a b Frank D. Gunstone, John L. Harwood, Albert J. Dijkstra: The Lipid Handbook. CRC Press, 2007, ISBN 0-8493-9688-3 , p. 7
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Johann Vollmann, Istvan Rajčan: Oil Crops. Springer, 2009, ISBN 978-0-387-77593-7 , p. 47 f.
- ↑ Entry on calendula oil. In: Römpp Online . Georg Thieme Verlag, accessed on May 3, 2019.
