Cycloheptane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cycloheptane | ||||||||||||||||||
| other names |
Heptamethylene |
||||||||||||||||||
| Molecular formula | C 7 H 14 | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 98.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.81 g cm −3 |
||||||||||||||||||
| Melting point |
−8 ° C |
||||||||||||||||||
| boiling point |
119 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4436 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cycloheptane is an organic compound that belongs to the group of substances called cycloalkanes . The connection can occur in various conformers .
Extraction and presentation
Cycloheptane occurs naturally in petroleum and can be extracted from it. A synthesis takes place via a Clemmensen reduction from cycloheptanone .
properties
Physical Properties
Cycloheptane is a colorless liquid with a mild, aromatic odor. The boiling point is 119 ° C at normal pressure . The heat of vaporization is 38.5 kJ mol −1 . According to Antoine, the vapor pressure function results according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.97710, B = 1330.402 and C = −56.946 in the temperature range from 341.3 K to 432.2 K.
In the solid phase, cycloheptane occurs in four polymorphic forms. The conversion temperatures for the conversion from Form IV to Form III are −138 ° C, from Form III to Form II at −75 ° C and from Form II to Form I at −61 ° C. Form I melts at −8 ° C.
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid | −156.4 kJ mol −1 | |
| Standard entropy | S 0 liquid | 242.55 J mol −1 K −1 | as a liquid |
| Enthalpy of combustion | Δ c H 0 liquid | −4598.9 kJ mol −1 | |
| Heat capacity | c p | 180.614 J mol −1 K −1 (25 ° C) 132.0 J mol −1 K −1 (25 ° C) |
as a liquid as a gas |
| Triple point | T triple | 265.12 K | |
| Critical temperature | T c | 604.2 K | |
| Critical pressure | p c | 38.2 bar | |
| Critical volume | V c | 0.353 l mol −1 | |
| Critical density | ρ c | 2.83 mol·l −1 |
Chemical properties
Cycloheptane can be thermally rearranged to methylcyclohexane in the presence of aluminum chloride .
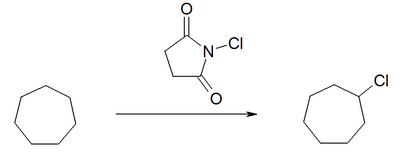
Functionalization can take place by chlorination with N -chlorosuccinimide .
The compound is flammable and forms flammable vapor-air mixtures with air. The flash point is 6 ° C, the lower explosion limit 1.1 vol .-%.
use
Cycloheptane can be used as a non-polar solvent. In organic synthesis, after functionalization, the cycloheptyl function can be converted into organic molecules such. B. pharmaceutical active ingredients are introduced.
Health hazards
An irritant effect on the eyes and respiratory tract is mentioned in the literature. The toxic effect is more comparable to that of methylcyclohexane , which only weakly irritates the mucous membranes. In animal experiments, there was only a slight irritative effect with regard to skin irritation. Systemically, cycloheptane has a depressive effect on the central nervous system .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry on cycloheptane in the GESTIS substance database of the IFA , accessed on March 29, 2018(JavaScript required) .
- ^ Brockhaus ABC chemistry. FA Brockhaus Verlag, Leipzig 1965, p. 1587.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-126.
- ↑ Entry on cycloheptane. In: Römpp Online . Georg Thieme Verlag, accessed on June 7, 2014.
- ↑ a b c d e f g H. L. Finke, DW Scott, ME Gross, JF Messerly, G. Waddington: Cycloheptane, Cyclooctane and 1,3,5-Cycloheptatriene . Low Temperature Thermal Properties, Vapor Pressure and Derived Chemical Thermodynamic Properties. In: J. Am. Chem. Soc. 78, 1956, pp. 5469-5476. doi: 10.1021 / ja01602a003 .
- ↑ ES Domalski, ED Hearing: Heat Capacities and Entropies of Organic Compounds in the Condensed phase. Volume III. In: J. Phys. Chem. Ref. Data . 25, 1996, pp. 1-525. doi: 10.1063 / 1.555985 .
- ↑ a b R. Spitzer, HM Huffman: The heats of combustion of cyclopentane , cyclohexane , cycloheptane and cyclooctanes . In: J. Am. Chem. Soc. 69, 1947, pp. 211-213. doi: 10.1021 / ja01194a006 .
- ↑ J.-L. Fortier, PJ D'Arcy, GC Benson: Heat capacities of binary cycloalkane mixtures at 298.15 K. In: Thermochim. Acta . 28, 1979, pp. 37-43, doi: 10.1016 / 0040-6031 (79) 87005-7 .
- ^ OV Dorofeeva: Thermodynamic properties of twenty-one monocyclic hydrocarbons. ( Memento of March 4, 2016 in the Internet Archive ) In: J. Phys. Chem. Ref. Data . 15, 1986, pp. 437-464. doi: 10.1063 / 1.555773 .
- ↑ a b c d T. E. Daubert: Vapor-Liquid Critical Properties of Elements and Compounds. 5. Branched Alkanes and Cycloalkanes. In: J. Chem. Eng. Data . 41, 1996, pp. 365-372. doi: 10.1021 / je9501548 .
- ^ S. Hauptmann, J. Graefe, H. Remane: Textbook of organic chemistry. German publishing house for basic industry, Leipzig 1980, p. 207.
- ↑ Ph. Buu-Hou, P. Demerseman: Halogenation of Saturated Compounds with N-Chloro- and N-Bromo-Succinimide. In: J. Org. Chem. 18, 1953, pp. 649-652. doi: 10.1021 / jo01134a005 .
- ↑ E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.