Cyclooctane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclooctane | |||||||||||||||
| other names |
Octamethylene |
|||||||||||||||
| Molecular formula | C 8 H 16 | |||||||||||||||
| Brief description |
colorless liquid with a musty odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 112.22 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.84 g cm −3 |
|||||||||||||||
| Melting point |
12-14 ° C |
|||||||||||||||
| boiling point |
150-152 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
almost insoluble in water (7.9 mg l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.4586 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
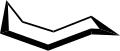
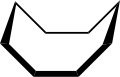
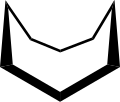
Cyclooctane is an organic-chemical compound that belongs to the group of cycloalkanes . The connection can occur in various conformers .
Presentation and extraction
The connection may be through a hydrogenation of 1,5-cyclooctadiene , which technically from 1,3-butadiene are produced is obtained.
properties
Cyclooctane is a faintly smelling, colorless liquid . The boiling point is 151.2 ° C at normal pressure . The heat of vaporization is 43.35 kJ mol −1 . The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.98805, B = 1438.687 and C = −63.024 in the temperature range from 369.9 K to 467.5 K. The critical values are 374 ° C for the critical temperature , 35.6 bar for the critical pressure , 0.4 l mol −1 for the critical volume and 2 for the critical density, 44 mol·l −1 . In the solid phase there are three polymorphic crystal forms. Polymorph III transforms into polymorph II at −106.6 ° C with a heat of transformation of 6.306 kJ mol −1 . At −89.4 ° C, the conversion of polymorph II to polymorph I then takes place with a heat of conversion of 0.478 kJ mol −1 . Polymorph I melts at 14.8 ° C with a heat of fusion of 2.410 kJ mol −1 . The heat capacity at 25 ° C is 215.53 J mol −1 K −1 or 1.92 J g −1 K −1 .
Cyclooctane forms highly flammable vapor-air mixtures. The compound has a flash point of 28 ° C. The lower explosion limit is 0.95% by volume. The ignition temperature is 250 ° C. The substance therefore falls into temperature class T3.
Conformations
Cyclooctane is representative of many figure eight rings. Its conformation has been studied intensively with the aid of a computer. Hendrickson noted that the "boat-chair" conformation is the most stable, which Allinger and his co-workers have confirmed. The “Crown” conformation is less stable.
Web links
Individual evidence
- ↑ a b c d e f g h i j k Entry on cyclooctane in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-130.
- ↑ Usha Rani; Muralidhar Reddy; Sarangapani; Ravinder: in J. Indian Chem. Soc. 84 (2007) 122-129.
- ↑ Muralidhar Reddy; Krista Reddy; Shobha Rani; Buchi Reddy Ravinder: in J. Indian Chem. Soc. 84 (2007) 733-738.
- ↑ Meyer, EF; Hotz, CA: Cohesive Energies in Polar Organic Liquids. 3. Cyclic Ketones in J. Chem. Eng. Data 21 (1976) 274-279, doi : 10.1021 / je60070a035 .
- ↑ a b c d Finke, HL; Scott, DW; Gross, ME; Messerly, JF; Waddington, G .: Cycloheptane , Cyclooctane and 1,3,5-Cycloheptatrienes . Low Temperature Thermal Properties, Vapor Pressure and Derived Chemical Thermodynamic Properties in J. Am. Chem. Soc. 78 (1956) 5469-5476. doi: 10.1021 / ja01602a003 .
- ^ Daubert, TE: Vapor-Liquid Critical Properties of Elements and Compounds. 5. Branched Alkanes and Cycloalkanes in J. Chem. Eng. Data 41 (1996) 365-372, doi : 10.1021 / je9501548 .
- ^ Wilhelm, E .; Faradjzadeh, A .; Grolier, J.-PE: Molar excess heat capacities and excess volumes of 1,2-dichloroethane + cyclooctane, + mesitylene, and + tetrachloromethane in J. Chem. Thermodyn. 11 (1979) 979-984, doi : 10.1016 / 0021-9614 (79) 90047-8 .
- ↑ Hendrickson James B .: Molecular Geometry V. Evaluation of Functions and Conformations of Medium Rings . In: J. Am. Chem. Soc. 1967, p. 7036-7043 , doi : 10.1021 / ja01002a036 .
- ↑ Dorofeeva, OV, Mastryukov, VS; Allinger, NL; Almenningen, A .: The molecular structure and conformation of cyclooctane as determined by electron diffraction and molecular mechanics calculations . In: The Journal of Physical Chemistry . 89, No. 2, 1985, pp. 252-257. doi : 10.1021 / j100248a015 .
- ↑ a b c d e P. W. Pakes, TC Rounds, HL Strauss: Conformations of cyclooctane and some related oxocanes . In: The Journal of Physical Chemistry . 85, No. 17, 1981, pp. 2469-2475. doi : 10.1021 / j150617a013 .
- ^ GP Moss: Basic terminology of stereochemistry (IUPAC Recommendations 1996) . In: Pure and Applied Chemistry . 68, No. 12, 1996, pp. 2193-2222. doi : 10.1351 / pac199668122193 .