Norepinephrine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Norepinephrine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 11 NO 3 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| solubility |
practically insoluble in water, ethanol and diethyl ether |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Noradrenaline or norepinephrine ( INN ) is an endogenous messenger substance that acts as a stress hormone and neurotransmitter . As a body hormone , the substance is produced in the adrenal medulla ; produced as a neurotransmitter in the nervous system (in the locus caeruleus ).
Norepinephrine is a catecholamine and is closely related to adrenaline . By narrowing blood vessels, it increases blood pressure. As the prefix Nor- indicates, unlike adrenaline, noradrenaline has no methyl group (-CH 3 ) on its amino group . Therefore, noradrenaline and adrenaline sometimes have different physiological effects.
| Catecholamines (comparison) |
|---|
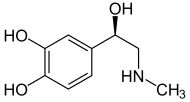 adrenaline |
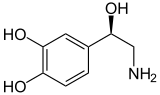 Norepinephrine |
history
Norepinephrine was discovered in 1948 by Peter Holtz , who at that time still called it norepinephrine. A short time later this discovery enabled the physiological effects of the two adrenal hormones (noradrenaline and adrenaline ) to be clarified. In 1949, M. Goldenberg introduced norepinephrine as a therapy for severe shock.
Acts as a hormone
In addition to adrenaline, noradrenaline is produced as a hormone in the adrenal glands and released into the blood (escape reflex). It mainly acts on the arterioles and, by activating adrenoceptors , constricts these vessels and therefore increases blood pressure .
Effect as a neurotransmitter
The most important function of norepinephrine is its role as a neurotransmitter in the central nervous system and the sympathetic nervous system . This distinguishes noradrenaline from adrenaline, which only has a subordinate neurotransmitter role.
Norepinephrine is released in the peripheral nervous system by sympathetic nerve fibers. It is a transmitter substance ( neurotransmitter ) of the post- ganglionic synapses of the sympathetic nervous system and has the same effect there as adrenaline . The elimination of noradrenaline from the synaptic cleft takes place mainly through re-uptake into the presynaptic cell via the transporter, but it can also be inactivated enzymatically. Norepinephrine reuptake inhibitors lead to an increase in the norepinephrine concentration and thus to an increase in the sympathetic tone .
In the locus coeruleus , a relatively small, dark-colored cell group in front of the fourth ventricle, a portion of the bridge (Pons), a large part of noradrenaline is of CNS produced. Benzodiazepines reduce the activity of the locus caeruleus and thus reduce the transport of noradrenaline to the forebrain .
Clinical information
biochemistry
Like adrenaline and dopamine , noradrenaline belongs to the group of catecholamines . Its natural stereoisomer is L - (-) - noradrenaline [synonym: ( R ) -noradrenaline], and its enantiomer D - (+) - noradrenaline [synonym: ( S ) -noradrenaline] is physiologically insignificant.
The production of norepinephrine takes place in the adrenal glands and in the nervous system from dopamine by means of the enzyme dopamine hydroxylase . Vitamin C plays a role as a cofactor and electron donor .
Pathological relevance
A pathologically increased concentration of noradrenaline in the blood is found in the clinical picture of heart failure .
Use as a medicinal substance
Norepinephrine is used as an emergency drug in intensive care medicine (in adults at a dose of 2–16 µg / min). It does a good job in the treatment of the following diseases:
It is usually administered intravenously using a syringe pump . Norepinephrine should be dosed as low as possible, as it makes it difficult for the heart to pump. The main target parameter of the dosage is adequate kidney excretion. Usually, norepinephrine is combined with a relatively large amount of fluid to fill up the intravascular volume.
Contraindications
Norepinephrine must not be used or only very carefully used in the following conditions:
- High blood pressure ( arterial hypertension )
- Cor pulmonale
- Angle-closure glaucoma
- Hyperthyroidism
- Pheochromocytoma
- severe arteriosclerosis with stenoses
- severe coronary sclerosis or severe heart muscle failure
- severe renal failure
- Supraventricular tachycardia
- Tachyarrhythmia
- Enlargement of the prostate with residual urine
Trade names
Monopreparations : Arterenol (D), as well as generic (CH)
Combination preparations : Scandonest (CH)
literature
- Hermann Voss , Robert Herrlinger (founder): Pocket book of anatomy. Volume 3: nervous system, sensory system, skin system, increment system. 17th, revised edition. Fischer, Stuttgart 1986, ISBN 3-437-00487-5 .
- Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , pp. 44-46.
Individual evidence
- ↑ a b c Entry on norepinephrine. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ^ A b Royal Pharmaceutical Society (ed.): Clarke's Analysis of Drugs and Poisons FOURTH EDITION . Pharmaceutical Press, London / Chicago 2011, ISBN 978-0-85369-711-4 .
- ↑ a b Entry on norepinephrine in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ^ Entry on noradrenaline in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 167 .
- ↑ RW Fuller: Pharmacology of brain epinephrine neurons . In: Annu. Rev. Pharmacol. Toxicol. . 22, 1982, pp. 31-55. PMID 6805416 .
- ↑ Jassal / D'Eustachio / reactome.org: Dopamine is oxidized to noradrenaline ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , pp. 44-46 and 76.
Web links
- Norepinephrine determination in urine ( Memento from September 23, 2012 in the Internet Archive )