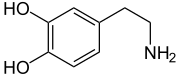
Dopamine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Dopamine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 11 NO 2 | |||||||||||||||||||||
| Brief description |
colorless prisms, with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 153.18 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
128 ° C |
|||||||||||||||||||||
| pK s value |
8.93 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dopamine ( DA , made up of DOPA and amine ) is a biogenic amine from the group of catecholamines and an important neurotransmitter of the central nervous system with a predominantly exciting effect . Dopamine is also called prolactostatin or PIH ( Prolactin-Inhibiting Hormone ). It is formed in (postganglionic sympathetic) nerve endings and in the adrenal medulla as a precursor of norepinephrine .
It is popularly known as the happiness hormone . The actual psychotropic significance of dopamine is assumed to be mainly in the area of drive increase and motivation.
Dopamine is also used as a drug , for example to treat cardiovascular shock .
Anatomy and physiology
Nerve cells that contain dopamine are called dopaminergic. Dopaminergic neurons are located in the central nervous system (CNS) and especially in the midbrain . The midbrain dopaminergic systems from major climb into the telencephalon and the diencephalon on. The associated neuron populations are found in the substantia nigra , in the area tegmentalis ventralis and in the retro-rubral regions. Dopamine is also a neurotransmitter in some systems of the autonomic nervous system and regulates the blood flow in internal organs. It is required for a large number of vital control and regulation processes.
Among other things, dopamine influences extrapyramidal motor skills (this may be related to Parkinson's disease ). The dopamine balance is also related to the neurobiology of psychoses and other disorders. Dopaminergic systems also intervene in the regulation of the hormonal balance. Dopamine from neurons that are located along the 3rd cerebral ventricle inhibits the release of the hormone prolactin in the pituitary gland . It also regulates the blood flow to the abdominal organs; dopamine in particular is involved in controlling the kidneys.
The effect of a dopamine release through a presynaptic termination on the postsynaptic neuron depends on the dopamine receptor type in the postsynapse. There are currently five dopamine receptors (D 1 –D 5 ). If dopamine binds to D 1 or D 5 , the downstream cell is depolarized ( excitatory postsynaptic potential ). Binding to receptors D 2 -D 4 causes hyperpolarization of the postsynapse ( inhibitory postsynaptic potential ). The latter types of receptors are collectively referred to as the D 2 group. There is also evidence that dopamine receptors of type D1 and type D2 can form so-called heterodimers , which leads to activation of the phospholipase signaling pathway and ultimately an increase in the intracellular Ca concentration. However, the physiological significance of this is still unclear.
There are essentially four dopaminergic processing pathways in the CNS:
- The mesostriatal system (also nigro-striatal system) has its origin in the substantia nigra in the midbrain and projects v. a. to the basal ganglia, which play an important role in controlling movement. This pathway is ascribed an essential role in the hypokinetic symptoms of Parkinson's disease and the frequently occurring extrapyramidal disorders as a side effect of neuroleptics .
- The mesolimbic system also originates in the area tegmentalis ventralis and projects v. a. to the limbic system (hippocampus, amygdala, corpus mamillare, fornix etc.). This path is very likely to be a major contributor to the so-called "positive" symptoms in schizophrenic disorders. It is considered to be the “reward system” , the reduction of which makes patients listless and listless ( anhedonia , often in Parkinson's patients ). In this system z. For example, intracranial self-stimulation, in which mice stimulate themselves through implanted electrodes until they are completely exhausted. Certain drugs, such as cocaine and amphetamines , also work on this system.
- The mesocortical system runs from the ventral tegmental area to the frontal lobe. According to current understanding, the functioning of this pathway has a meaning for the so-called executive functions , as well as the motivation. In connection with psychoses of the schizophrenic form , an underactivity is seen here that is associated with the cognitive disorders that are often associated with these diseases.
- The tuberoinfundibular system, whose neurons run from the arcuate nucleus to the anterior pituitary gland, where they inhibit the release of prolactin .
Dopamine is ascribed an important role in addiction diseases . The use of various intoxicating drugs increases the effects of dopamine, serotonin and gamma-aminobutyric acid . The disturbance in the dopamine level is responsible for some of the withdrawal symptoms.
In ADHD , there may be a lack of dopamine (predominantly) in the striatum . The typical ADHD drugs (stimulants such as methylphenidate or amphetamines ) cause an increase in the dopamine level in the synaptic gap in various ways (MPH as a dopamine reuptake inhibitor, amphetamine drugs as a dopamine release enhancer) - with the correct dosage to the level that ensures interference-free signal transmission, as with those who are not affected . Overdosing (too high dopamine levels) causes signal transmission problems that cause almost the same symptoms as too low dopamine levels.
biosynthesis
Dopamine is an intermediate product in the biosynthesis of adrenaline from the amino acid tyrosine . Tyrosine is converted to levodopa by tyrosine hydroxylase and this in turn to dopamine by aromatic L-amino acid decarboxylase .
Medical use
Dopamine lowers the peripheral resistance through vasodilation and thus increases the cardiac output and thus the glomerular filtration rate . However, the therapeutic use of dopamine as catecholamine in states of shock , very low blood pressure or kidney failure is increasingly taking a back seat, since in addition to obvious undesirable effects such as cardiac arrhythmias , immunosuppression and endocrinological disorders occur, which are particularly common in the treatment of critically ill patients Critical care medicine are problematic.
For the treatment of Parkinson , which is due to the destruction of cells in the substantia nigra to a deficiency of dopamine in the basal ganglia is coming, and the restless legs syndrome is levodopa (L-DOPA), a prodrug of dopamine given. Dopamine itself would not get into the central nervous system because it can not cross the blood-brain barrier . Dopamine is formed from L-DOPA in the basal ganglia via decarboxylation (see figure). This would happen even before it flows into the CNS, which is why it is necessary to combine L-DOPA with a substance that inhibits the enzyme responsible for decarboxylation ( aromatic L-amino acid decarboxylase ) . Usually takes place a galenical processing of the L-DOPA to a combination preparation with a decarboxylase inhibitor such as carbidopa or benserazide . Since neither carbidopa nor benserazide, in contrast to L-DOPA, can cross the blood-brain barrier, the conversion to dopamine in the CNS is not blocked.
Dopamine hypothesis of schizophrenia
According to the dopamine hypothesis , an excessively high dopamine level in certain areas of the brain has been associated with the symptoms of schizophrenia ( psychoses ) since the 1960s . The use of highly dopaminergic substances ( pramipexole , amphetamine ) can trigger corresponding symptoms even in healthy people.
Dosage forms, finished medicinal products
The water-soluble dopamine hydrochloride is used medicinally . It is only used intravenously with an indication-dependent dosage of 2–30 µg / kg / min.
As a finished medicinal product , dopamine is usually sold as an infusion solution concentrate under its generic name , in Austria there is also a preparation called Giludop or Revivan in Italy.
Starting substance for alkaloid synthesis in plants
Among other things, dopamine is a building block in the biosynthesis of isoquinoline alkaloids, e.g. B. Berberine . In animal tissue, too, the biosynthesis of morphine from dopamine takes place via the same intermediate stages ( laudanosine , reticulin and thebaine ) as in plants. This could be demonstrated using various cell cultures.
See also
literature
- Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York a. a. 1999, ISBN 3-540-65024-5 , p. 47 f.
- Renate Frank (Hrg): Therapy goal well-being: Activating resources in psychotherapy . Springer, Berlin, Heidelberg 2017, ISBN 978-3-662-53470-0 .
Web links
- Ilka Lehnen-Beyel: Confusion among brain messenger substances . In: Wissenschaft.de, April 8, 2005: "When using antidepressants, the balance between serotonin and dopamine gets mixed up."
- Dopamine modulation . In: Scholarpedia . (English, including references)
Individual evidence
- ↑ a b c Entry on dopamine. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c Entry on dopamine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Datasheet Dopamine hydrochloride from Sigma-Aldrich , accessed on March 28, 2011 ( PDF ).
- ↑ UConn Researcher: Dopamine Not About Pleasure (Anymore) , UConn Today.
- ↑ SP Lee, CH So u. a .: Dopamine D1 and D2 Receptor Co-activation Generates a Novel Phospholipase C-mediated Calcium Signal. In: Journal of Biological Chemistry. 279, 2004, p. 35671, doi: 10.1074 / jbc.M401923200 .
- ↑ Practice book dopamine disorders, diagnosis and therapy . Sandoz AG, Nuremberg no year, p. 9.
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992, ISBN 3-927408-82-4 . P. 94
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York a. a. 1999, ISBN 3-540-65024-5 , pp. 44-48 and 76.
- ↑ GW. Kirby: Biosynthesis of the morphine alkaloids . In: Science , 1967 Jan 13, 155 (3759), pp. 170-173, PMID 5332945
- ↑ Chotima Poeaknapo, Jürgen Schmidt, Matthias Brandsch, Birgit Dräger, Meinhart H. Zenk: Endogenous formation of morphine in human cells . In: PNAS (Proc Natl Acad Sci USA) , 2004 Sep 28; 101 (39), pp. 14091-14096, PMC 521124 (free full text).

